- Course Overview
- Module 1: Introduction
- Module 2: Preliminary Assessments
- Module 3: Assisted Reproduction Treatments
- Module 4: Ovulation Induction
- Module 5: Intrauterine Insemination
- Module 6: IVF and ICSI
- Module 7: Risks and Complications
- Module 8: Pregnancy-related Complications
- Module 9: Perinatal and Paediatric Outcome
- RCOG Assisted Reproduction Technology (ART) – Complete Assessment Guide
- Course Overview
- Assessment 1 – Assisted Reproduction (5/5 – 100%)
- Final Assessment 1 (25/25 – 100%)
- Final Assessment 2 (4/4 – 100%)
- Final Assessment SBAs (8/8 – 100%)
- Assessment: Part 3 – IUI Suitability (1/1 – 100%)
- Assessment: Part 4 – Prognostic Factors (2/2 – 100%)
- Assessment: Part 5 – OHSS (2/2 – 100%)
- Assessment: Part 3 – Tubal Patency (2/2 – 100%)
- Assessment: Part 4 – Clomiphene Citrate (2/2 – 100%)
- Key Learning Points Summary
- Reflective Tasks
Course Overview
This document contains all topics from the RCOG Assisted Reproduction eLearning course.
Last updated: December 2025
—
Module 1: Introduction
Topic 1: Introduction
Assisted Reproductive Technology (ART) is a blanket term used to describe technologies that help a person or couple conceive with the assistance of medical intervention. Examples of assisted reproduction are:
- Intrauterine insemination (IUI)
- In vitro fertilisation (IVF) – one of the most commonly used and successful treatments available
- IVF with intracytoplasmic sperm injection (ICSI)
- The use of donor sperm (donor insemination) or eggs (egg donation) or embryos (embryo donation)
- Vitrification
The first pregnancy after the fertilization of a human egg in vitro and the first birth from an in vitro-fertilized embryo were reported in 1976 and 1978, respectively. Since then, more than five million pregnancies have been achieved worldwide by IVF and its modifications, known generically as assisted reproductive technologies (ARTs). As experience has accumulated, success rates have increased, and the indications for these procedures have expanded, such that ART now accounts for 1 to 3 percent of live births in Europe and the United States.
The average age of first-time mothers across the EU increased from 27.6 years in 2006 to 30.2 years in 2022, and the mean age of childbearing has been increasing consistently in Europe since the 1980s. Women under 30 have an 85% chance of conceiving within one year, compared to 66% by age 35 and 44% by age 40, and fertility decreases dramatically after this.
Regulatory Framework
In response to concerns about how rapidly the technologies were developing, in 1991 the UK created the Human Fertilisation and Embryology Authority (HFEA) through the Human Fertilization and Embryology Act 1990 (HFE Act). This was the first statutory body of its kind in the world. The HFEA regulates and inspects all UK fertility clinics and is dedicated to licensing and monitoring the fertility clinics and all UK research involving human embryos, and providing impartial and authoritative information to the public.
The introduction of guidelines into clinical practice in general and in assisted reproduction in particular has led to an ever-increasing role for an evidence-based approach to the provision of these services. Clinical guidelines for the assessment and treatment for people with infertility problems were published by the National Institute for Clinical Excellence in 2004 and later updated in February 2013 to streamline services and avoid unnecessary waste of resources. The impact of the HFEA, the Royal College of Obstetricians and Gynaecologists (RCOG) and National Institute for Health and Care Excellence (NICE) publications on the management of the infertile couple has been considerable on UK practice.
Learning Outcomes
When you have completed this course you will be able to:
- Outline the suitability of assisted reproduction treatments for the different diagnostic categories
- Explain the principles of assisted reproduction treatments and their associated psychological stresses
- Describe the complications associated with assisted reproduction treatments and their management
- Summarise the outcomes and success of the different treatments
- Identify pregnancy-related problems following assisted reproduction treatments
- Identify fetal and neonatal complications following assisted reproduction treatments
RCOG Statement
Within this course we use the terms woman and women’s health. However, it is important to acknowledge that it is not only women for whom it is necessary to access women’s health and reproductive services in order to maintain their gynaecological health and reproductive wellbeing. Gynaecological and obstetric services and delivery of care must therefore be appropriate, inclusive and sensitive to the needs of those individuals whose gender identity does not align with the sex they were assigned at birth.
Certificate
In order to receive the Royal College of Obstetricians and Gynaecologists certificate for this course, please ensure that you complete all of the final assessments.
—
Topic 2: Contributor Profiles
Authors:
- Dr Archana Ranganathan (2025)
Editorial Board Members:
- Dr Rachel Squires (2023-2026)
- Dr Snigdha Veeramalla (2023-2026)
Contributors:
- Deepthi Lavu (2019; Author)
- Kanna Jayaprakasan (2019; Author)
- Dr Nikoletta Panagiotopoulou (2021-2023; Editorial Board)
- Dr Alexandros Grammatis (2020-2023; Editorial Board)
- Deepthi Lavu (2018-2021; Editorial Board)
- Kanna Jayaprakasan (2018-2021; Editorial Board)
- Mona El-Talatini (2016; Author)
- Hemlata Thackare (2014; Peer-reviewers)
- Richard T Russell (2010; Peer-review)
- Shaheen Khazali (2009; Trainee reviewer)
- Nazar Amso (original author)
- Lyndon Miles (original author)
—
Topic 3: Essential Reading
Essential Reading:
- National Institute for Health and Care Excellence. Fertility: Assessment and Treatment for People with Fertility Problems. CG156. London: NICE; 2013. (https://www.nice.org.uk/guidance/cg156)
- Mascarenhas M, Oliver J, Bhandari HM. Routes to parenthood for women with Turner syndrome. The Obstetrician and Gynaecologist 2019;21:43–50.
Further Reading:
- Royal College of Obstetricians and Gynaecologists. The Effect of Surgery for Endometriomas on Fertility. RCOG Scientific Impact Paper No. 55. RCOG; 2025.
- The Human Fertilisation and Embryology Authority. Homepage. HFEA.
- Bonduelle M, Wennerholm UB, Loft A, Tarlatzis BC, Peters C, Henriet S, et al. A multi-centre cohort study of the physical health of 5-year-old children conceived after intracytoplasmic sperm injection, in vitro fertilization and natural conception. Hum Reprod 2005;20:413–9.
- Verhaak CM, Smeenk JM, Evers AW, Kremer JA, Kraaimaat FW, Braat DD. Women’s emotional adjustment to IVF: a systematic review of 25 years of research. Hum Reprod 2007;13:27–6.
- Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Warner L, et al. Assisted reproductive technology surveillance — United States, 2012. Surveill Summ 2015; 64:1.
- Kupka MS, Ferraretti AP, de Mouzon J, Erb K, D’Hooghe T, Castilla JA, et al. Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHRE. Hum Reprod 2014; 29:2099.
- Kovacs, G, editor. How to Improve your ART Success Rates. 1st ed. Cambridge University Press; 2011.
Other Resources:
- eJournals, eBooks and Databases. Royal College of Obstetricians and Gynaecologists.
Note: The essential reading includes papers suggested by the authors for trainees to read before they begin working through an eTutorial. They are not compulsory. Papers listed under further reading are articles relating to the topic, but may not be available as full-text and are for further information only.
—
Topic 4: Related CiPs
The content of this course relates to the following Capabilities in Practice (CiPs) and key skills:
CiP 1: Clinical skills and patient care
- Relevant to all key skills
CiP 9: Emergency gynaecology and early pregnancy
- Manages complications of treatment
CiP 11: Non-emergency gynaecology and early pregnancy
- Manages subfertility
- Manages abnormal vaginal bleeding
CiP 12: Non-emergency obstetrics
- Manages conditions arising in pregnancy
CiP 13: Non-discrimination and inclusion
- Mental and physical health associations
Further details about each CiP can be found within the O&G Core Curriculum 2024 Definitive Document.
Module 2: Preliminary Assessments
Assessment 1 – Assisted Reproduction
Score: 5/5 – 100%
For each of the following clinical scenarios choose the most appropriate treatment option.
—
Question 1: A couple where the male partner is healthy and the female partner suffers from Turner’s syndrome (XO)
Answer: In vitro fertilisation using donor oocytes ✓
Feedback: The answer is in vitro fertilisation using donor oocytes.
—
Question 2: A couple with no male factor problem but the female partner has polycystic ovarian syndrome not responding to clomifene and metformin therapy
Answer: In vitro fertilisation using own gametes ✓
Feedback: The answer is in vitro fertilisation using own gametes.
—
Question 3: A couple with unexplained infertility
Answer: In vitro fertilisation using own gametes ✓
Feedback: The answer is in vitro fertilisation using own gametes.
—
Question 4: A couple where the female partner has pelvic endometriosis affecting the tubes and the male partner has severe oligo-astheno-spermia and is a carrier of cystic fibrosis gene mutation
Answer: Intracytoplasmic sperm injection ✓
Feedback: The answer is intracytoplasmic sperm injection. This can be done with the woman’s own egg, provided she is not a carrier of CF.
—
Question 5: A couple where the female partner has complete Rokitansky syndrome with right renal absence
Answer: Surrogacy ✓
Feedback: The answer is surrogacy. In Rokitansky syndrome, the uterus is missing and not ovaries so patient can use her own eggs.
Module 3: Assisted Reproduction Treatments
Topic 1: Clinical Indications for Assisted Reproductive Treatments
Assisted reproduction treatment (ART) are indicated in the following scenarios:
Indications Related to Subfertility
- Tubal factor infertility – IVF directly bypasses the fallopian tubes
- Male factor infertility
- Ovulatory dysfunction – for example PCOS – failure to respond to or conceive with ovulation induction medications
- Reduced ovarian reserve or ovarian failure (with donor eggs)
- Unexplained infertility
- Gestational carrier/Surrogacy – in patients for whom pregnancy is relatively contraindicated or with uterine factor infertility
- Coital dysfunction
Indications Not Related to Subfertility
- Single women, same sex couples seeking fertility treatment
- Preimplantation genetic testing (known carriers of certain genetic disorders or parental translocations)
- Fertility preservation – such as prior to gonadotoxic therapy or patients desiring to delay childbearing
—
Topic 2: Prognostic Factors and Patient Selection
1. Female Age
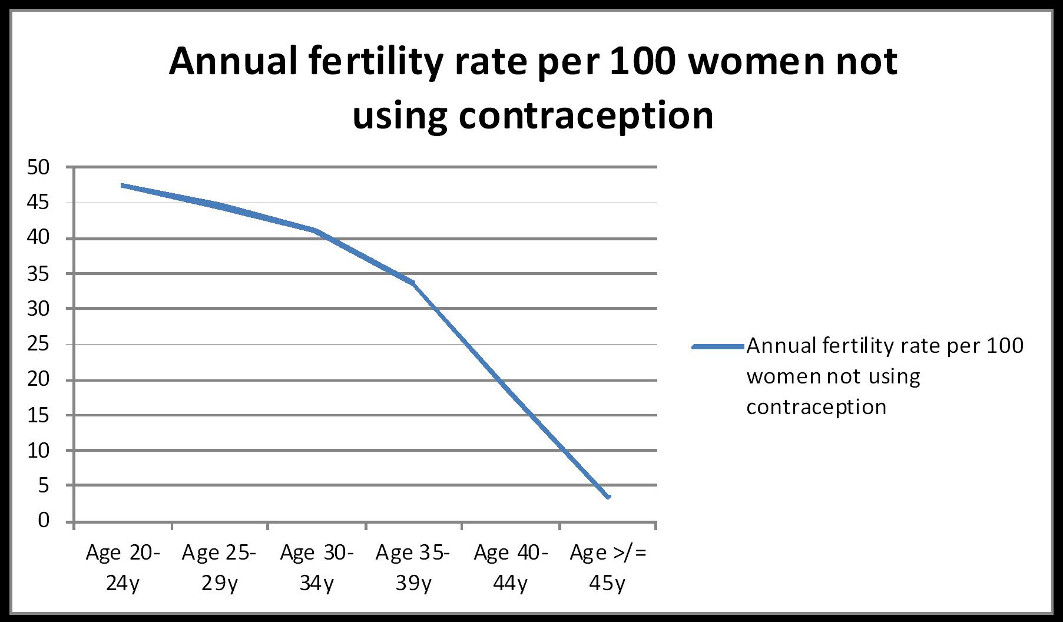
Fertility naturally declines with age due to decreasing ovarian reserve and oocyte quality and accelerates after 35 years of age. This decline leads to:
- Decreased fertility
- Delayed conception
- Increased miscarriage rates
- Higher incidence of chromosomal abnormalities
A woman’s age is the most significant predictor of successful outcome, and the chances of a live birth after IVF treatment vary with age.
NICE Recommendations:
- Offer three cycles of IVF to women younger than 40 years who have not conceived after 2 years of unprotected intercourse
- The use of IVF beyond the age of 40 years is associated with a reduction in the chance of conception
- Evidence suggests a significant decrease in age-adjusted live birth rates with increasing duration of infertility between 1 and 12 years
2. Ovarian Reserve
Assessment of ovarian reserve has been used to predict the likelihood of a successful response to ovarian stimulation with ART. Several tests are used:
- FSH levels – Early follicular phase FSH correlates well with response to ovarian stimulation (though inter-cycle variability is high)
- AFC (Antral Follicle Count) – Using ultrasound, an effective predictor of ovarian stimulation
- AMH (Anti-Mullerian Hormone) – High accuracy in assessing ovarian reserve; produced by granulosa cells in preantral and antral follicles; can be measured at any time with low inter-cycle variability
NICE Guidelines for Predicting Ovarian Response:
| Parameter | Low Response | High Response |
|---|---|---|
| AFC | ≤4 | >16 |
| AMH | ≤5.4 pmol/l | ≥25 pmol/l |
| FSH | >8.9 IU/l | – |
3. Past Reproductive History and Previous Fertility Treatment
- Women who have been previously pregnant, particularly those with live birth, have a significantly higher probability of a live birth compared with those with no previous pregnancies
- The overall chance of a live birth following IVF treatment falls as the number of unsuccessful cycles increases
4. Body Mass Index (BMI)
- A BMI outside the range of 19 to 29 is likely to be associated with reduced success of assisted reproduction procedures
- BMI ≥30 and not ovulating: Losing weight is likely to increase chance of conception
- BMI <19 with irregular/absent menstruation: Increasing body weight is likely to improve chance of conception
5. Semen Quality
- Semen parameters (sperm count, motility, morphology) significantly influence IVF success rates
- WHO 2023 report: Male factor infertility is involved in about 50% of infertility cases, either as a primary or contributing cause
—
Topic 3: Patient Assessment and Screening Tests Before Treatment
It is important to obtain a detailed history and to assess the couples who are undergoing IVF prior to starting treatment. It is also essential to assess welfare of the child issues, offer counselling and discuss available support options.
Pre-treatment Requirements
- Couples should be given appropriate information regarding the nature of the treatment and its risks
- Written consent should be obtained for the use and storage of gametes and embryos as stipulated by the HFEA
- Women should be offered assessment of their rubella status
- Chlamydia trachomatis screening or prophylactic antibiotic treatment should be considered prior to uterine instrumentation
- Couples should be screened for hepatitis B, hepatitis C and HIV to assess risk for cross contamination before processing gametes
General Health and Dietary Advice
| Factor | Recommendation |
|---|---|
| Alcohol | Women trying to get pregnant should avoid drinking alcohol. Men ❤ units per day (excessive intake is detrimental to sperm quality) |
| Smoking | Negative effect due to nicotine on ovarian, uterine and placenta function. Association between smoking and reduced sperm quality in men |
| Folic acid | Supplementation advised before conception and for up to 12 weeks of gestation to reduce risk of fetal neural tube defect |
| Caffeine | Current evidence does not suggest association between caffeine consumption and infertility problems |
—
Topic 4: Key Points
Careful couple assessment prior to undergoing ART treatment is important in order to allow them to start treatment in their most optimal condition. This will identify and help the management of any medical condition that might affect the chance of success as well as pose a risk in pregnancy to mother or fetus.
Module 4: Ovulation Induction
Topic 1: Ovulation Induction
Medical therapy is often effective in women with ovulatory dysfunction.
WHO Group I Ovulation Disorders
Women with WHO group I ovulation disorders (hypothalamic pituitary failure, amenorrhoea or hypogonadotrophic hypogonadism) should be offered:
- Pulsatile gonadotrophin-releasing hormone (GnRH), or
- Gonadotrophins with luteinising hormone
These are effective in inducing ovulation.
Hyperprolactinaemia
- Women with hyperprolactinaemia should be referred to an endocrinologist to exclude a pituitary micro- or macroadenoma
- Can be treated medically with dopamine agonists such as:
- Bromocriptine
- Cabergoline
Weight Management
- Women should be advised to maintain their weight between a BMI of 19 and 29 to optimize their natural fertility and response to infertility treatments
- NICE suggests funding for assisted conception should be restricted to patients who maintain their weight between these parameters
—
Topic 2: Polycystic Ovarian Syndrome (PCOS)
The most common cause of hypothalamic pituitary dysfunction (WHO group II) resulting in anovulatory infertility is polycystic ovary syndrome (PCOS). Medical treatment aims to induce unifollicular development.
First-Line Therapy: Antiestrogens
NICE guidelines advise the use of antiestrogens (clomifene citrate or tamoxifen) for up to 6 months as first-line therapy:
- Clomifene increases pregnancy rate in PCOS patients compared with placebo (OR: 5.8)
- Clomifene is as effective as tamoxifen
- Cumulative pregnancy rates continue to rise after six treatment cycles before reaching a plateau
- Ultrasound monitoring of at least one cycle helps assess response and may necessitate dosage adjustment
Adverse effects of antiestrogens:
- Hot flushes
- Ovarian hyperstimulation syndrome (OHSS)
- Abdominal discomfort
- Multiple pregnancies
Letrozole
A Cochrane review showed that letrozole appears to improve live birth rates and pregnancy rates compared to clomifene citrate when used for ovulation induction followed by timed intercourse. No difference in miscarriage rate or multiple pregnancy rate.
Clomifene-Stimulated IUI
Women who ovulate with clomifene but do not conceive may be offered clomifene-stimulated intrauterine insemination, before proceeding to IVF if necessary.
Metformin
- Improves clinical pregnancy and ovulation rates
- Does NOT improve live birth rates when used alone or with clomifene
- With IVF: Does not increase live birth rate but reduces incidence of OHSS
- Side effects: Nausea, vomiting, gastrointestinal disturbances
- Dosing: Start 500 mg once daily → 500 mg twice daily → max 1 g twice daily (if tolerated)
Laparoscopic Ovarian Drilling
For women unresponsive to first-line treatments:
- As effective as gonadotrophin treatment
- Not associated with increased risk of multiple pregnancies
- Associated with surgical risks
- Benefits wane with time
- Long-term effects poorly understood
Gonadotrophins
- Human menopausal gonadotrophin (hMG), urinary FSH, or recombinant FSH (r-FSH) are equally effective
- Consider minimising cost when prescribing
- Growth hormone treatment with GnRH agonist and/or hMG does NOT improve pregnancy rates
Risks and Counselling
- Risks of multiple pregnancy and OHSS must be highlighted before treatment
- Ovarian ultrasound monitoring should be integral to patient management
- Possible association between ovulation induction therapy and ovarian cancer (remains uncertain)
Module 5: Intrauterine Insemination
Topic 1: Intrauterine Insemination (IUI)
Intrauterine insemination (IUI) entails timely insemination of sperm into the uterus in either:
- Unstimulated cycle (where the woman is ovulatory), or
- Stimulated cycles (where the woman is anovulatory) using oral anti-estrogens (clomiphene citrate, tamoxifen) or gonadotrophin injections
IUI in stimulated cycles are undertaken even in ovulatory women with the hypothesis that superovulation (multiple follicle development) may increase the probability of pregnancy.
Requirements for IUI
- Ovulation in the IUI cycle
- Patency of at least one Fallopian tube
- Insemination with an adequate number of motile sperm
- Absence of active cervical, intrauterine, or pelvic infection
IUI Technique Steps
- Oral anti-estrogens (clomiphene/tamoxifen): tablets taken for 5 days
- Gonadotrophins: subcutaneous injections for 7-10 days
- Ultrasound monitoring of ovarian response
- When 1-3 follicles developed with dominant follicle >17 mm: hCG injection to trigger ovulation
- Insemination 24-36 hours after hCG trigger
- If >2 ovulatory follicles present: insemination cancelled to reduce multiple pregnancy risk
Indications for IUI
- Unexplained infertility
- Endometriosis (minimal, mild)
- Mild male factor
- Cervical factor infertility
- Physical or psychological disability preventing penetrative vaginal intercourse
- Conditions requiring specific consideration (e.g., sperm washing in HIV positive male)
- As part of donor insemination
Guideline Recommendations
NICE 2013 (updated 2017):
- For unexplained infertility, mild endometriosis or mild male factor: do NOT routinely offer IUI
- Exceptions: social, cultural or religious objections to IVF
- Advise trying to conceive for total of 2 years before IVF
ESHRE 2023 (Unexplained infertility):
- Recommends ovarian stimulation with IUI (OS-IUI) as first-line treatment
- Use low-dose gonadotropins to avoid OHSS and multiple pregnancies
ESHRE 2022 (Endometriosis):
- May perform IUI with ovarian stimulation in rASRM stage I/II endometriosis
Success Factors
- Age of woman
- Number of developing follicles
- Endometrial thickness
- Duration and type of infertility
- Progressive motility of sperm sample
Risks of IUI
| Risk | Details |
|---|---|
| Upper genital tract infection | Rare potential complication |
| Ectopic pregnancy | Unclear if risk increased |
| Multiple gestation | Significantly increased with gonadotropins; much lower with clomiphene |
| OHSS | Usually only with gonadotropin cycles following hCG; rare with clomiphene |
—
Topic 2: Key Points
- IUI is recommended for various clinical indications, although NICE 2017 recommendations restrict its use to indications other than unexplained infertility, mild male factor and mild endometriosis
- IUI can be offered as first-line treatment for couples with unexplained infertility and for those with mild (stage I–II) endometriosis, as it improves pregnancy rates compared to natural cycles or expectant management
- IVF is reserved for cases with failed IUI, advanced maternal age, or moderate-to-severe endometriosis
- IUI can be performed in a natural unstimulated cycle or a stimulated cycle
- The most important risk associated with stimulated IUI is multiple pregnancy and the treatment is ideally cancelled if there is multi-follicular response
Module 6: IVF and ICSI
Topic 1: IVF and ICSI – Overview
In vitro fertilization (IVF) is a type of assisted reproductive technology (ART) where an egg is fertilized by sperm outside the body (in vitro), typically in a laboratory setting.
General Process:
- Ovaries are stimulated by a combination of fertility medications
- Oocytes are aspirated from ovarian follicles via transvaginal ultrasound-guided follicle aspiration
- Oocytes are fertilized in vitro
- Embryos are cultured in the laboratory
- One or more embryo(s) transferred into the uterine cavity
Intra-cytoplasmic sperm injection (ICSI) differs from conventional IVF in that the embryologist selects a single sperm to be injected directly into an egg, instead of fertilisation taking place in a dish where many sperm are placed near an egg.
Indications for IVF and ICSI Treatments
Although IVF was initially introduced to bypass tubal blockage, the currently available techniques enable the treatment of various causes of infertility. The current indications are:
- Tubal disease
- Male factor infertility – which is not correctable through other measures
- Endometriosis – where other treatments have been deemed unsuitable or been unsuccessful
- Unexplained infertility – in women where other less invasive treatments have been unsuccessful
- Ovulatory disorders – where other options have been exhausted or unsuccessful
- Egg donation – for women with premature ovarian failure or poor oocyte quality
- ICSI specifically indicated – when there is severe impairment of sperm quality or number, obstructive or non-obstructive azoospermia, and where previous IVF treatment was accompanied by failed or very poor fertilisation
- Embryo donation or surrogacy
- Embryo testing:
- PGT-M: Monogenic disorders
- PGT-SR: Structural chromosomal rearrangements
- PGT-A: Aneuploidy screening
- Fertility preservation – for cancer patients or non-medical reasons
- Same sex couples and single women – IVF with donor sperm when other approaches have failed or are not suitable (Malizia et al, 2009)
—
Topic 2: Key Components of IVF Treatment Pathway (Procedures)
The treatment cycle involves a number of principal steps that include:
- Pre-treatment evaluation
- Controlled ovarian stimulation – using gonadotrophins and GnRH analogues (agonists or antagonists)
- Monitoring follicular development – using transvaginal ultrasound with or without serum estradiol levels
- Oocyte maturation – using hCG or GnRH agonist trigger
- Egg collection and sperm production or sperm recovery
- Fertilization (IVF/ICSI) and subsequent embryo culture
- Fresh embryo transfer into the uterus and cryopreservation of surplus good quality embryos
- Luteal support through progesterone administration
—
Topic 3: Pretreatment Evaluation
Emphasis should be placed on the pre-treatment evaluation of the individual or couple both medically and psychologically. The assessment of the welfare of the child that may result from ART has become central to the provision of treatment in the eyes of the HFEA.
Key Considerations:
- Careful consideration of the duration and stability of the couple’s relationship
- Medical and social backgrounds and other relevant issues
- Information-giving and decision-making aspects of counselling should be made available by a specially trained counsellor
- All couples should be interviewed together with appropriate history and clinical examination for both individuals
Required Tests for Female Partner:
- Baseline hormone profile
- Mid-luteal phase progesterone assay
- Rubella status
- Hepatitis B and C and HIV screening (mandatory in UK before ART for both partners)
- HTLV in some instances
Factors Affecting IVF Success:
- Age
- Ovarian reserve
- Cause and duration of infertility
- Past reproductive-obstetrical history
- Presence or absence of any pelvic pathology
Age
The age of the woman is a major determinant of the success of IVF as both qualitative and quantitative ovarian reserve declines with advancing age.
Ovarian Reserve
A woman’s fertility is related to the number of oocytes remaining in her ovaries (‘ovarian reserve’), which influences her chance of becoming pregnant. Evaluation of ovarian reserve is an integral part of pre-treatment assessment.
Aims:
- Identify women likely to respond poorly (low chance of success, more likely to have cycle cancelled)
- Identify those prone to ovarian hyperstimulation syndrome (OHSS)
- Allow treatment protocol to be tailored to the individual
Tests for Ovarian Reserve (NICE CG156, 2013):
| Test | Low Response | High Response |
|---|---|---|
| FSH | > 8.9 IU/l | – |
| Total AFC | ≤ 4 | > 16 |
| AMH | ≤ 5.4 pmol/l | > 25.0 pmol/l |
Pelvic Pathology
Ultrasound evaluation of the pelvis is often performed prior to IVF to rule out adnexal and uterine pathologies such as tubal disease, fibroids, endometrial polyps and endometriosis.
Hydrosalpinx
- Associated with poor implantation, low pregnancy rates and early pregnancy loss
- Substances toxic to the endometrium are thought to negatively affect endometrial receptivity
- Diagnosis can generally be made using transvaginal ultrasound
- Surgical treatment should be considered for all women with hydrosalpinges prior to IVF
- Laparoscopic salpingectomy or proximal tubal occlusion prior to embryo transfer is recommended to improve pregnancy rates
Leiomyoma (Fibroids)
| Type | Effect on IVF | Recommendation |
|---|---|---|
| Submucosal (FIGO 0-2) | Significantly reduced implantation (RR=0.283), clinical pregnancy (RR=0.363), ongoing pregnancy/live birth (RR=0.318); increased miscarriage | Should be surgically removed |
| Intramural | Unclear but may have adverse effects | Consider removal |
| Subserosal | No effect | Do not need removal |
Endometrial Polyps
- Effect on IVF outcomes remains unclear
- Some studies suggest polyps < 2cm have no impact
- Reasonable to remove prior to treatment (provides histological sample and may improve outcome)
Endometrioma and Endometriosis
- Operative laparoscopy for stage I/II endometriosis improves ongoing pregnancy rates
- Routine surgery prior to IVF for stage I/II is NOT recommended (evidence unclear)
- Surgery for ovarian endometrioma is NOT routinely recommended (associated with reduced ovarian reserve)
Consider surgery if:
- Severe pelvic pain or symptoms attributable to the cyst
- Rapid growth or atypical ultrasound features raising malignancy concern
- Very large cyst(s) preventing follicle access or oocyte retrieval
- Recurrent endometrioma after prior surgery causing significant issues
- Patient preference after informed consent (with counselling about ovarian reserve loss)
Previous Pregnancy History
- A previous live birth is associated with higher likelihood of successful IVF
- History of one or more miscarriages does NOT reduce likelihood of success
Previous Unsuccessful IVF Cycle
- Lack of success in an IVF cycle does not decrease success rates during subsequent treatment until approximately the fourth IVF cycle
Obesity
- IVF success may be affected in women with increased BMI
- Losing weight to achieve optimum BMI is advised
Smoking
- Smoking in both women and men can affect fertility and IVF success adversely
- Couples should be advised to stop smoking
—
Topic 4: Controlled Ovarian Stimulation
Ovarian stimulation protocols first used clomiphene citrate with or without gonadotrophin, which often led to difficulty in timing of oocyte retrieval. With time, more complex regimens have evolved using a variety of agents. The two most common protocols used in practice are the long cycle and the short cycle.
Drugs Used in Ovarian Stimulation
GnRH Agonists (e.g. buserelin, nafarelin)
- Synthetic GnRH agonist that competitively blocks the action of GnRH
- Prevents release of LH and FSH from the anterior pituitary gland
- Initially there is a release of FSH and LH – the ‘flare effect’
GnRH Antagonists (e.g. cetrotide)
- Competitively and reversibly bind to GnRH receptors in the pituitary gland
- Block release of LH and FSH from the pituitary, preventing ovulation
- Rapid acting and do NOT have a flare effect
Gonadotrophins
- Urinary-derived: Human menopausal gonadotrophin (hMG) or FSH (e.g. Menopur®, Fostimon®)
- Often have a small component of LH
- Recombinant: rFSH (e.g. Puregon®, Gonal F®)
- Considered pure (without LH component)
- Very little data demonstrates superiority of synthetic over urinary preparations; consider cost
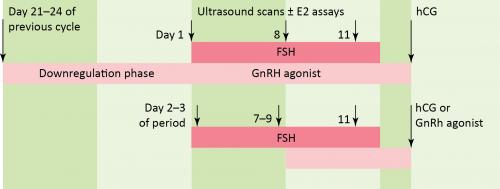
“Long protocols” involve starting medications in the menstrual cycle before the IVF cycle; this is often done with a GnRH agonist. By contrast, “Short protocols” are regimens in which medications are started at the start of the menstrual cycle in which IVF is performed. Stimulation is achieved with human menopausal gonadotropins (hMG) or FSH and spontaneous ovulation is blocked with either a GnRH agonist (by using the initial stimulation of leuprolide) or with a GnRH antagonist. GnRH antagonists are preferred over GnRH agonists for the short protocol.
The Long Protocol
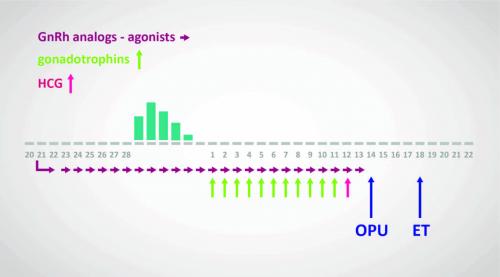
- Utilises GnRH agonist for 2–3 weeks to desensitise the pituitary
- Down regulation is confirmed by:
- Thin endometrium
- Lower plasma estradiol level (not routinely used if endometrium thin and ovaries quiescent)
- Ovarian stimulation commenced using hMG or rFSH with USS follicular monitoring
- GnRH agonist continued concurrently with stimulation
- hCG or recombinant LH administered to induce final maturation of oocytes
- Oocyte collection arranged for 34–37 hours after final hCG administration
The Short Protocol
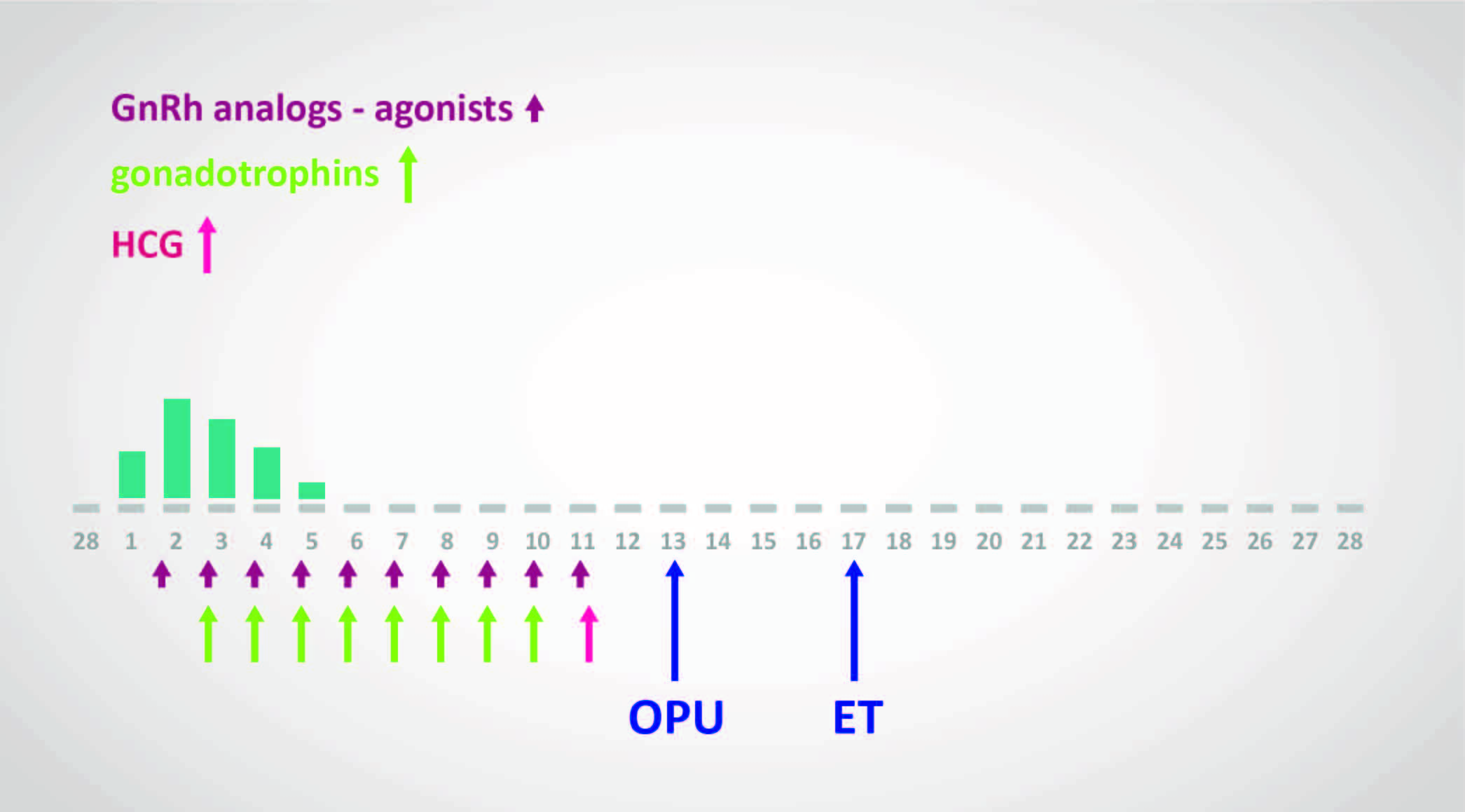
- No downregulation stage to desensitise the pituitary
- hMG or rFSH administered in early menstrual phase (usually day 2 or 3)
- GnRH antagonists administered from day 5 or 6 of stimulation
- Used concurrently with gonadotrophins
- Prevents premature luteinisation prior to follicle maturity
- Continue until follicles reach appropriate stage of growth on monitoring
- hCG or recombinant LH administered to induce final maturation of oocytes
- Oocyte collection arranged for 34–37 hours after final hCG administration
Note: “Long protocols” involve starting medications in the menstrual cycle before the IVF cycle (often with GnRH agonist). “Short protocols” start medications at the start of the menstrual cycle in which IVF is performed. GnRH antagonists are preferred over GnRH agonists for the short protocol.
—
Topic 5: Controlled Ovarian Stimulation – Part 2
Short GnRH Antagonist Protocol Benefits
- Shorter duration of treatment
- Avoidance of pituitary down regulation
- Lower risk of OHSS with similar rates of fertilisation
- On average, one less egg is obtained per woman per oocyte collection
Indications for GnRH Antagonist Protocol:
- Patients at high risk of OHSS
- Previous poor responders to treatment
- Patients requiring timely treatment (e.g., fertility preservation)
Major Advantage: Reducing risk of OHSS using GnRH agonist trigger (induces endogenous LH surge instead of hCG trigger) if exaggerated ovarian response – virtually eliminates risk of severe OHSS.
Low-Dose and Mild Stimulation IVF
- Recent transition to low-dose stimulation in IVF patients
- Evidence suggests improved pregnancy outcomes and reduced OHSS risks
- Natural cycle or mild stimulation IVF reduces costs and risks further
- Requires more cycles to achieve pregnancy
- UK implementation limited by funding provisions
Monitoring Follicular Development
Purpose:
- Ensure safe practice in reducing incidence and severity of OHSS
- Optimise timing of luteinisation before oocyte retrieval
Monitoring Protocol:
- Ultrasound monitoring is integral part
- Follicular response and endometrial thickness monitored by USS from day 6-8 of treatment cycle
- Serum estradiol levels may be measured (but current evidence does not support this as routine)
- Gonadotrophin dosage adjusted according to ovarian response
Typical Response:
- Follicles grow at rate of 2-3 mm/day
- Steady increase in serum estradiol levels
- When ≥3 follicles are >17-18 mm diameter → hCG trigger administered
hCG Trigger Options:
- 5000-10000 U hCG subcutaneously, OR
- 250-500 mcg choriogonadotropin alfa (recombinant)
Ovarian Response Issues
Poor Response:
- Definition: ❤ follicles develop after 14 days of gonadotrophin treatment
- Generally results in cancellation of stimulation cycle
- Frequently encountered in women with predicted reduced ovarian reserve
Excessive Response:
- May result in OHSS
- Other problems (premature LH surge, preoperative ovulation) largely eliminated by GnRH analogues
—
Topic 6: Analgesia and Anaesthesia for Oocyte Retrieval
Effective analgesia is essential during trans-vaginal oocyte retrieval, as the procedure is inherently painful.
Analgesia Options
| Option | Notes |
|---|---|
| IV Conscious Sedation | Standard of care, most commonly offered |
| IV Analgesia | If sedation not suitable or patient prefers |
| Local Analgesia (Para-cervical block) | Alternative option |
| General Anaesthesia | Reserved for those who cannot tolerate sedation; rarely offered |
Timing and Indications
- Oocyte retrieval typically performed 34-37 hours after hCG or recombinant LH trigger injection
- Historical method: laparoscopic collection (now rarely required)
- Current standard: Ultrasound-guided trans-vaginal aspiration
- Well tolerated
- Low morbidity
- Quick recovery
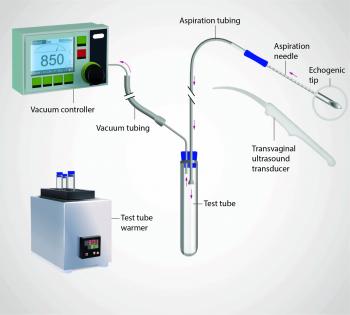
Equipment
- Transvaginal ultrasound transducer with needle guide
- Single- or double-lumen aspiration needle (usually 16-gauge)
- Tubing system connecting needle to collection tube
- Suction pump or manual aspiration device
- Sterile probe cover and protective sheath
Procedure
- Trans-vaginal ultrasound transducer fitted with sterile cover and needle guide
- 16-18 gauge aspiration needle (sharp tip with roughened distal segment for USS visibility) introduced through vaginal wall
- Needle connected via tubing to collection test tube and suction system
- Follicles aspirated sequentially under real-time ultrasound guidance
- If follicle doesn’t yield oocyte, flushing with pre-warmed culture media can be attempted (dual-lumen needle)
- Note: Benefit of follicle flushing remains controversial; repeated flushing may reduce fertilisation rates
Risks and Complications
| Complication | Incidence | Notes |
|---|---|---|
| Pelvic infection | ~0.6% | Higher risk in endometriosis or previous PID; routine prophylactic antibiotics not supported by evidence but may consider when puncturing endometriomas |
| Bleeding | ~0.8% | Vaginal or intraperitoneal bleeding >100 mL; rarely significant haemorrhage |
| Organ injury | Uncommon | Bowel, bladder, ureteric, tubal, uterine, or vascular trauma documented |
Risk Reduction: Careful vaginal preparation and minimising repeated passes with the needle
Identification of Oocytes
- Most oocytes from antral follicles will be at metaphase II
- Eggs from smaller follicles may be at various stages between prophase I and metaphase II (no polar body)
- Theoretically, one egg recovered from each follicle
- Oocytes at retrieval are encased in granulosa cells called the cumulus complex
- Granulosa cells possess FSH receptors
- Gonadotrophin stimulates growth and estrogen production
- Granulosa cells communicate with egg during development and maturation
Maturity Assessment:
- Cumulus expansion
- Corona cell dispersion
- Coronal association with zona pellucida
Topic 7: ICSI Technique
Conventional IVF
- Fertilisation conditions optimised by controlling sperm concentration
- Typically 100,000–200,000 motile sperm per oocyte required for adequate fertilisation
- Minimises multinucleate fertilisation
- Minimises oocyte exposure to damaging reactive oxygen species from sperm cells
ICSI Procedure
- Oocytes assessed for maturity by removing surrounding cumulus cells
- Presence of polar body ascertained
- Single sperm with normal morphology and progressive motility selected
- Sperm injected directly into mature oocyte using a micromanipulator
Advantages:
- Avoids need for large numbers of motile sperm
- Bypasses most sperm-related barriers to fertilisation
Indications for ICSI
- Suboptimal semen parameters
- Poor history of fertilisation
- Surgical sperm retrieval (e.g., azoospermia) – often the only feasible fertilisation method
- Cryopreserved (vitrified) oocytes – due to zona changes after vitrification
Fertilisation Rates
- Expected around 60–70% for ICSI
Evidence: ICSI vs IVF in Non-Male Factor Infertility
A systematic review and meta-analysis (Cutting et al, 2023) of RCTs comparing ICSI vs IVF in non-male factor infertile patients found:
- ICSI reduced risk of total failure of fertilisation
- ICSI increased overall fertilisation rate per oocyte
- ICSI did NOT significantly increase live birth rate
—
Topic 8: Laboratory Techniques
Sperm Collection Methods
| Method | Description |
|---|---|
| Masturbation | Standard method |
| PESA | Percutaneous Epididymal Sperm Aspiration (for azoospermia) |
| TESA | Testicular Sperm Aspiration (for azoospermia) |
| TESE | Testicular Sperm Extraction (for azoospermia) |
| Micro TESE | Better success rate in extracting sperm compared to standard single biopsy |
Insemination Techniques
Numerous techniques vary from laboratory to laboratory:
- Quantity and volume of sperm added to oocytes varies
- Oocytes can be cultured singly or in groups
- Open or oil-covered culture system can be employed
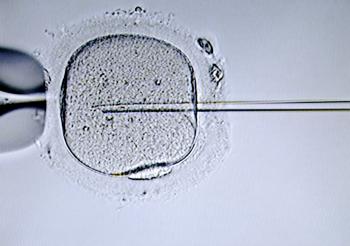
ICSI Process
- Meiotic maturity assessed after denudation
- Only mature oocytes injected
- Sperm is immobilised before injection
Fertilisation Assessment
- Fertilisation determined by presence of two pronuclei (2 PN)
- Assessment at 18-20 hours post-insemination
Embryo Transfer Timing
| Stage | Timing |
|---|---|
| Cleavage stage | Day 2 or Day 3 |
| Blastocyst stage | Day 5 |
- Transfer performed with one or two best available embryo(s) regardless of grading
- Only top quality embryos considered eligible for freezing
Advanced Sperm Selection Techniques
PICSI (Physiological Intracytoplasmic Sperm Injection)
- Uses a functional test to select sperm
IMSI (Intracytoplasmic Morphologically Selected Sperm Injection)
- Uses high-magnification microscopy for visual assessment
Note: Robust evidence establishing significant improvement in live birth rates with either PICSI or IMSI is currently limited. They are not routinely recommended (Boulet et al, 2015).
—
Topic 9: Early Embryonic Development
Signs of Fertilisation
Normal Fertilisation:
- Presence of two pronuclei (2PN) within the ooplasm
- Visible for a finite time period (12–21 hours post-insemination)
- Essential to perform rigorous scrutiny prior to pronuclei dissolution
- All abnormally fertilised eggs must be discarded
Abnormal Fertilisation:
| Finding | Indication |
|---|---|
| One pronucleus | Spontaneous activation of oocyte without fertilisation |
| Three pronuclei | Typically: two sperm fertilising the egg (IVF), or failure of emission of second polar body (digynic fertilisation), or rarely injection of diploid sperm cell (ICSI) |
Embryo Selection
Istanbul Consensus (ESHRE, 2025):
- Morphological sub-classification of pronuclei (including nucleolar patterning) lacks predictive value
- Should NOT be used for embryo selection
Historical markers (no longer recommended):
- Polar body position
- Pronuclei alignment
- Distribution of nucleoli within pronuclei
Fertilisation Benchmark
Approximately 50-70% of mature (MII) oocytes are expected to show normal (2PN) fertilisation under standard IVF/ICSI conditions.
Time-Lapse Monitoring
- Continuous monitoring allows tracking of nucleolar dynamics
- Allows exact timing of appearance and fading of pronuclei
- Forms basis for emerging AI-driven selection tools
- Considered an add-on for embryo selection (robust data demonstrating clear live-birth benefit still limited)
—
Topic 10: Assisted Hatching
Indications
May be recommended for:
- Patients with history of failed IVF/ICSI attempts who have good embryo quality
- Older patients (>38 years)
- Patients with elevated FSH
- Those with thickened or abnormal-looking zona pellucida
Procedure
- On morning of Day 3, best embryos are selected
- Small hole made in zona using:
- Small bore pipette filled with acid Tyrode’s solution, OR
- Laser
- Embryos cultured for a few hours
- Subsequently replaced in patient’s uterus
Evidence
- Hypothesised that hole in zona increases embryo’s implantation potential
- No strong, consistent evidence that assisted hatching significantly increases live birth rates in IVF/ICSI
Risk
- Increased risk of monozygotic twins (Lacey et al, 2021)
—
Topic 11: Embryo Transfer – Day of Transfer and Technique
Embryo Grading
Day 2/3 (Cleavage Stage) Embryos:
- Day 2: approximately 4 cells
- Day 3: approximately 8 cells
- Grading criteria:
- Degree of fragmentation
- Cell symmetry
- Granularity
- Vacuolisation
- Membrane definition (compaction)
Day 5 (Blastocyst Stage) Embryos:
- Grading criteria:
- Cavitation
- Expansion
- Zona thinning
- Presence of inner cell mass
- Hatching
(Glujovsky et al, 2022)
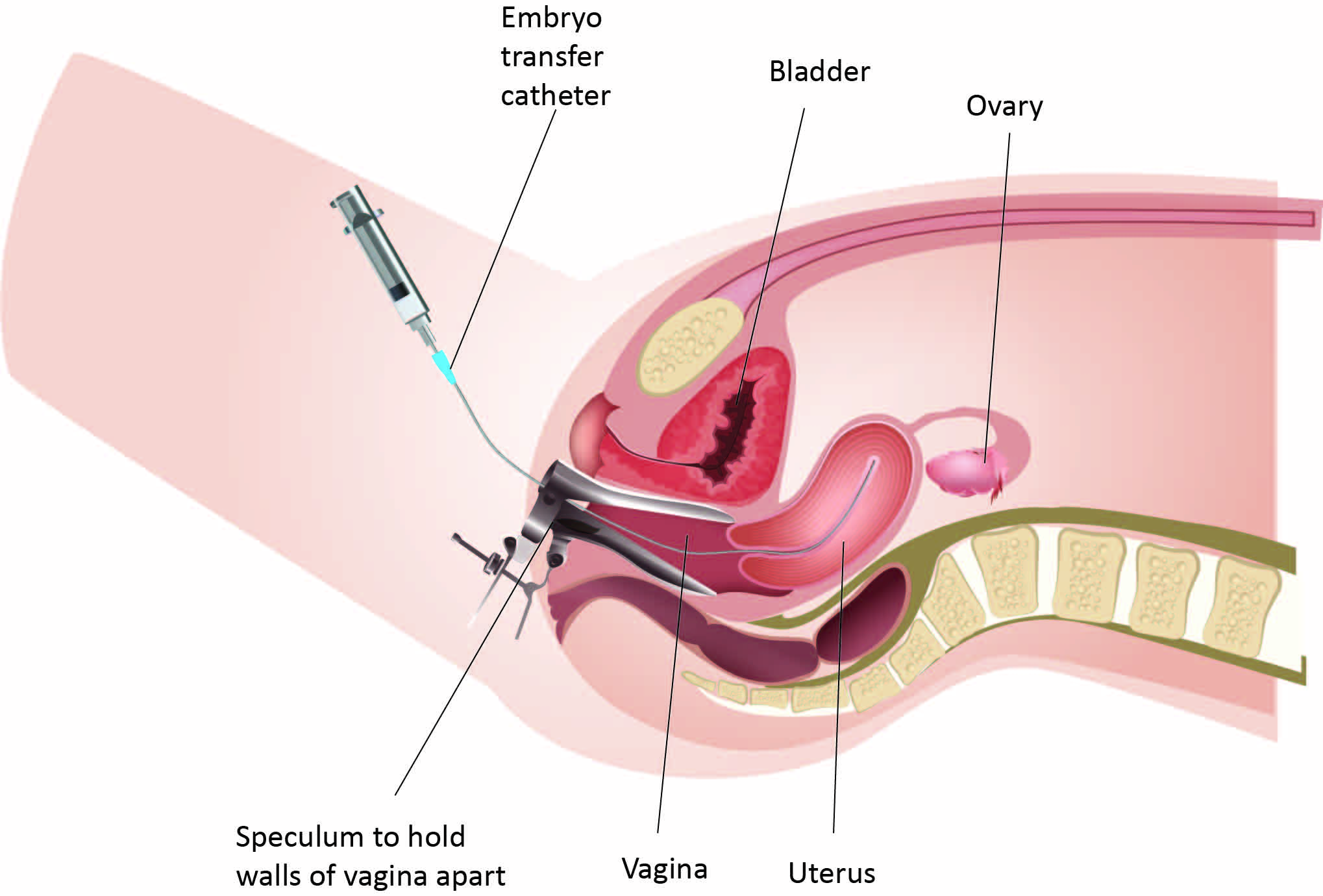
Transfer Technique
- Transfers typically done at blastocyst stage, occasionally cleavage stage
- Usually without need for sedation
- Embryos introduced into uterine cavity using small bore silicone catheter
- Passes transcervically
Ultrasound-Guided Transfer (Recommended)
- Associated with improved pregnancy rates
- Key techniques:
- Careful uterine placement (1–2 cm from fundus)
- Gentle handling
- Minimising uterine contractions
- Atraumatic transfer with soft catheter
MIP Point (Maximal Implantation Potential):
- 3D ultrasound-guided transfer precisely into mid-cavity
- Suggested to further improve results compared to “blind”/clinical-touch transfers
—
Topic 12: Embryo Transfer – Part 2
Modern IVF Practice: Blastocyst Single Embryo Transfer
Modern practice has moved decisively towards blastocyst single embryo transfer – balancing success rates and patient safety.
Rationale for Blastocyst-Stage Transfer:
- Extended culture offers more reliable assessment of embryo competence
- Higher implantation potential in appropriately selected patients
- Improves uterine and embryonic synchronicity
- Enables self-selection of viable embryos
- Results in better live birth rates
Evidence: Cochrane review (Glujovsky et al, 2022) demonstrated that transferring blastocysts results in higher live birth rates than day-3 transfer.
Elective Single Embryo Transfer (eSET)
- Markedly lowers incidence of multiple pregnancy
- Does NOT compromise cumulative live birth outcomes when high-quality embryos available
- Large systematic review (Ma et al, 2022): 95% reduction in multiple pregnancy risk with SET
Enabling Factors:
- Advancement in embryo culture media
- Better vitrification techniques
- Excellent post-thaw success rates
UK Policy and HFEA Regulations
- Single-embryo transfer policy in effect in many UK units
- HFEA target: reduce multiple birth rate to no more than 10%
Current Practice:
- No more than two embryos transferred per embryo transfer (standard)
- No more than three embryos only in exceptional circumstances
- Transfer of three embryos must be reported to HFEA on individual named patient basis
(Cutting et al, 2009)
—
Topic 13: Luteal Phase Support
Rationale
- Pituitary desensitisation and aspiration of granulosa cells at oocyte collection prevents adequate progesterone production
- Results in luteal phase deficiency
- Progesterone supplement optimizes endometrial receptivity
Timing of Initiation
- Fresh cycles: Within two days of oocyte retrieval
- Frozen embryo transfer (FET) cycles: Depends on protocol:
- Natural FET: Usually ~36 hours after LH surge
- Stimulated or programmed cycles: Protocol-specific timing
Duration
- Optimum duration NOT established
- Given until positive or negative pregnancy test
- Or until end of first trimester
Routes of Administration
| Route | Preparations |
|---|---|
| Intramuscular | Progesterone injection |
| Vaginal | Suppositories, tablets, gel, or ring |
Note: Intramuscular and vaginal preparations are equally effective
Safety
- Progesterone preparations and doses are NOT associated with:
- Birth defects
- Virilisation
HCG for Luteal Phase Support
NOT recommended – either alone or with progesterone:
- Not more effective than progesterone alone
- Increases risk of OHSS
Mild Stimulation Approaches
- Gaining interest in IVF field
- Aim to minimise adverse effects of ovarian stimulation (OHSS, multiple pregnancies)
—
Topic 14: Success Rates
Source: HFEA data published June 2025
UK IVF Statistics (2023)
- 52,400 patients had over 77,500 IVF cycles at licensed UK fertility clinics
- Babies born from IVF: ~8,700 (2000) → 20,700 (2023)
- IVF births as proportion of all UK births: <1.5% (2000) → >3% (2023)
Pregnancy and Birth Rates (Fresh Embryo Transfers, 2023)
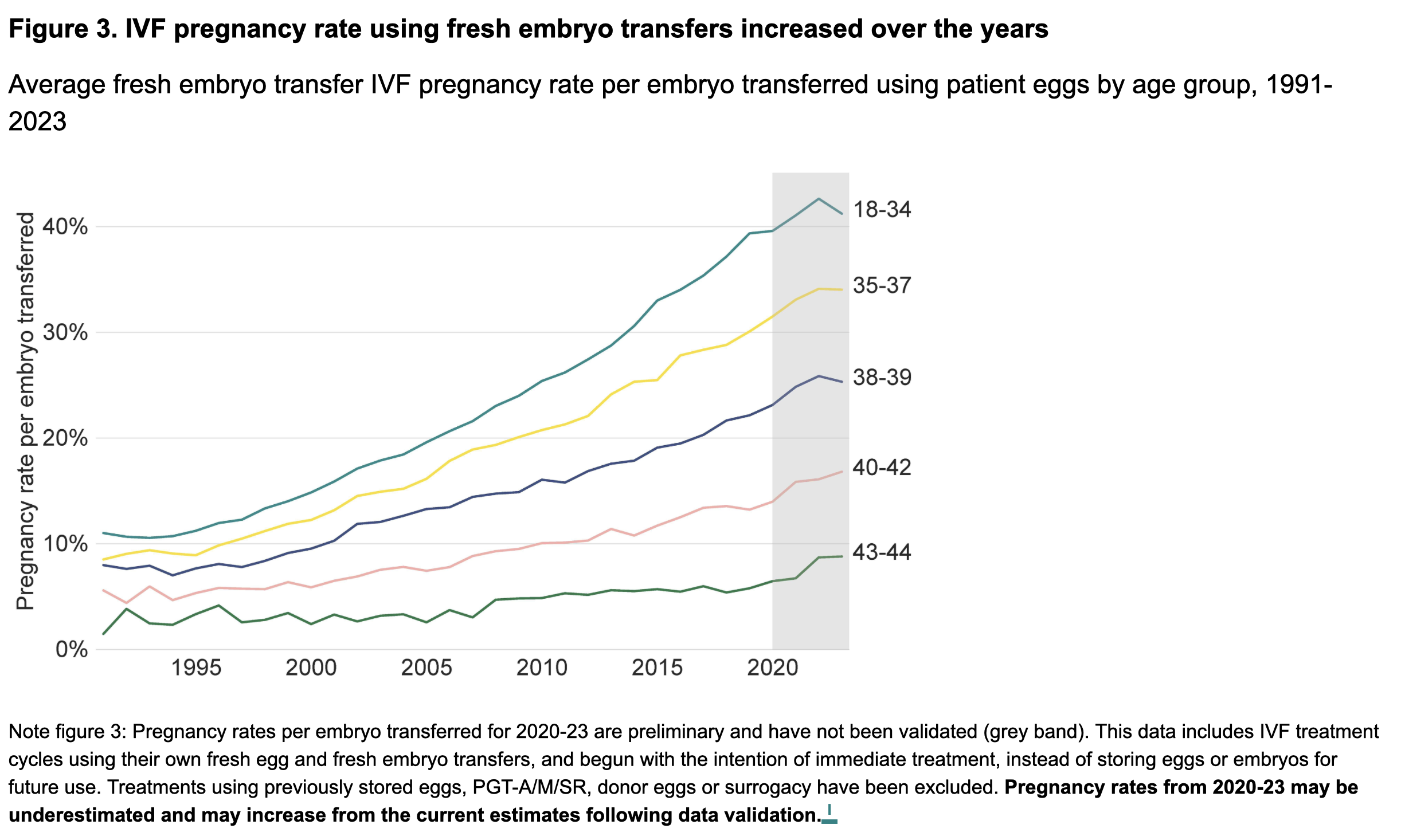
| Measure | Rate |
|---|---|
| Average pregnancy rate | 31% per embryo transferred |
| Highest pregnancy rate (18-34 years) | 41% |
| Average birth rate | 25% nationally |
Birth Rates by Age (Own Eggs, 2023)
| Age Group | Birth Rate |
|---|---|
| 18-34 years | 35% |
| 43-44 years | 5% |
—
Topic 15: Success Rates – Part 2 (Clinical and Lifestyle Factors)
Clinical Factors Affecting Success
Duration of Infertility:
- Increasing duration significantly decreases live birth rate irrespective of age
Infertility Factors:
| Factor | Outcome |
|---|---|
| Male or multiple factors | Lowest outcomes |
| Tubal, endocrine, unexplained | Comparable success rates to natural conception in young fertile couples |
| Hydrosalpinges | Benefit from pre-IVF salpingectomy |
| Secondary infertility | Higher pregnancy and live birth rates than primary infertility |
Female Age:
- Pregnancy and live birth rates decrease with advancing age
- Optimal treatment age: 23-38 years
- Lowest pregnancy rates: women aged ≥40 years
- Age affects outcome irrespective of fresh or frozen embryos
Treatment Cycles:
- Each IVF attempt has similar success rate to others
- With increased cycles, advancing female age determines outcome
- May observe lower pregnancy and live birth rates over time
Alternative Techniques:
- No evidence that TET/ZIFT (tubal embryo/zygote transfer) or GIFT (gamete transfer) results in higher pregnancy or live birth rates
Inter-Centre Variation:
- Differences in success rates exist between various centres
Lifestyle Factors Affecting Success
| Factor | Recommendation |
|---|---|
| Alcohol | >1 unit/day reduces effectiveness of ART |
| Smoking | Negative effect on outcome in either partner |
| Caffeine | No conclusive evidence of adverse effects; recommend moderating (1-2 cups coffee/day) |
| BMI | NICE recommends BMI 19-30 before commencing fertility treatment (likelihood of success reduced outside this range) |
—
Topic 16: Key Points – IVF and ICSI
- Pre-treatment evaluation of the couple is essential, as it allows treatment to be tailored to maximise the chances of a successful outcome
- Aims of superovulation regimens:
- Maximise the number of follicles
- Minimise degree of asynchrony amongst developing follicles
- Minimise deleterious effects of abnormal follicular environment on luteal function and endometrial receptivity
- Fully informed consent must emphasise potential outcomes, risks, costs, and handling of unused embryos (disposal, donation to research, or donation to other couples)
- Emotional and psychological toll of ART is significant:
- Implication counselling can help patients manage stress, anxiety, and potential disappointment
- Can improve overall mental health and potentially outcomes
—
Topic 17: Preimplantation Genetic Diagnosis (PGD)
Definition
PGD is performed on cell(s) removed from a preimplantation embryo or a polar body from an oocyte.
Indications
- Monogenic disorders
- Chromosomal rearrangements (e.g., translocations)
- Medically indicated gender selection – where a genetic condition only affects one gender or affects one gender more severely
- HLA-matched sibling – to save a sick child
Important: When PGD is planned, ART must be used for conception even if infertility is not an issue for the couple.
Procedure Steps
Embryology Component:
- Creating and culturing embryos until sufficient cells available for biopsy
- Biopsy of embryo using micromanipulation techniques
- One or two cells removed and isolated for genetic analysis
Genetic Analysis Methods:
| Genetic Defect | Analysis Method |
|---|---|
| Translocations and chromosome-linked defects | Fluorescent in situ hybridization (FISH) |
| Single-gene defects | PCR techniques |
Confirmation
- Small but non-zero rate of false-positive and false-negative results
- Chorionic villus biopsy or genetic amniocentesis recommended to confirm PGD findings
Evidence on PGD Screening
Current evidence does NOT support the use of preimplantation genetic screening to:
- Improve live birth rates, OR
- Reduce miscarriage rates in ART cycles
(Particularly for women of advanced age)
(Dahdouh et al, 2015; Sermon et al, 2004)
—
Topic 18: Oocyte Donation
Oocyte donation sits at the crossroads of advanced assisted reproduction science, legal and regulatory obligations, ethical complexities, and deeply personal choice.
Indications
- Advanced maternal age (age-related fertility decline)
- Genetic disease risk mitigation (e.g., cystic fibrosis, thalassemia, sickle cell)
- Premature ovarian insufficiency (primary or secondary)
- Poor ovarian reserve or oocyte quality
- After gonadotoxic therapy or oophorectomy
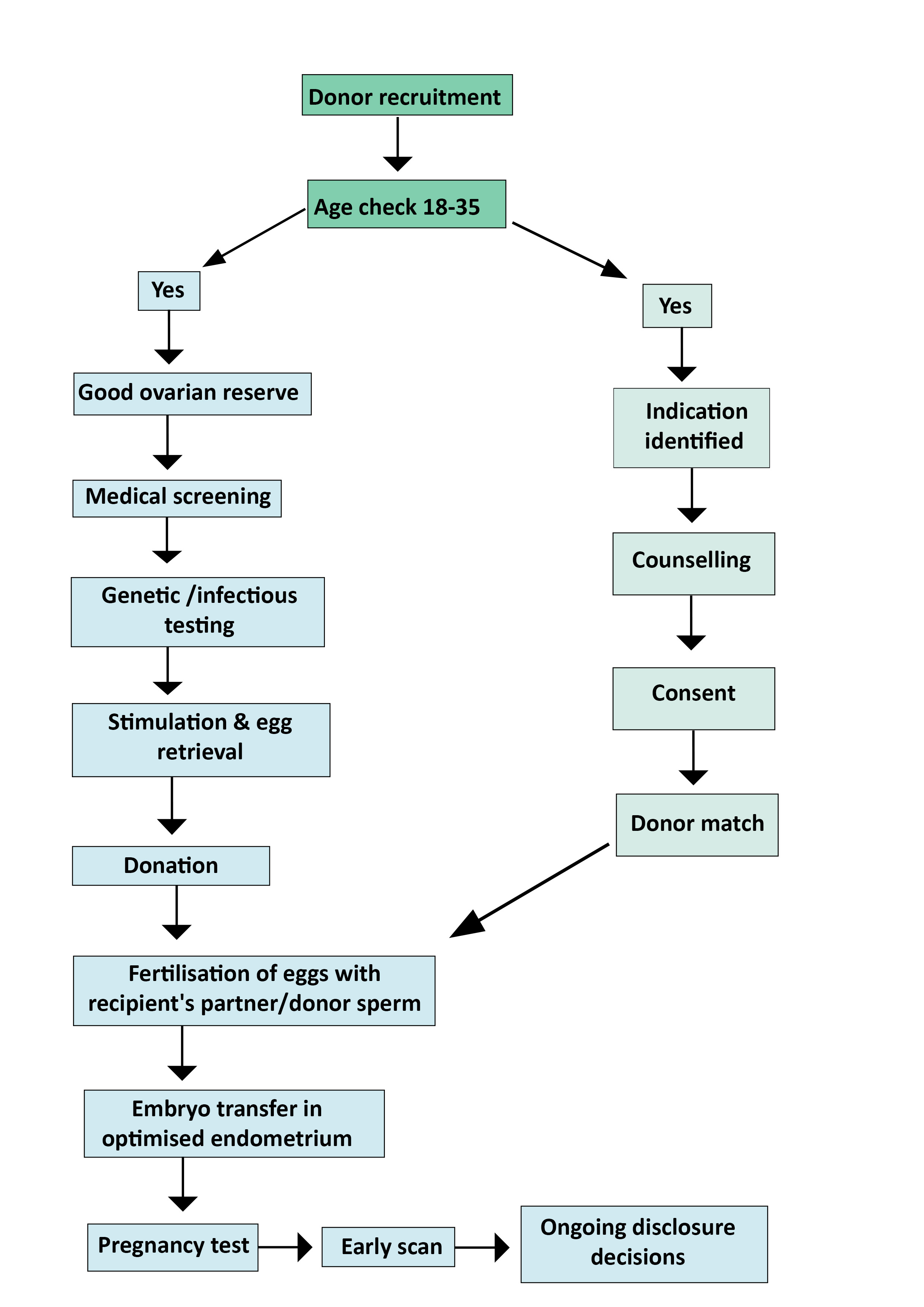
UK Regulatory Timeline
| Year | Milestone |
|---|---|
| Late 1980s | First UK baby from donor eggs |
| 1990 | Human Fertilisation and Embryology Act established |
| 1991 | HFEA established (world’s first fertility regulator) |
| Pre-2005 | Donors were anonymous by law |
| 2005 | Anonymity removed – donors must agree to identity-release |
| 2012 | Compensation rules updated: £750 per egg donation cycle |
| 2023 | First cohort of donor-conceived adults (born after 2005) turned 18 and could request identifying donor info; HFEA launched #WhoIsMyDonor campaign |
| Present | UK faces persistent donor shortages; increased importation of donor gametes |
Information Access for Donor-Conceived Children
- At age 16: Non-identifying info (physical traits, ethnicity, medical history)
- At age 18: Full identifying info (name, DOB, last known address)
Success Rates
- Pregnancy rates typically 50–60% (dependent on donor age and fertility)
- Reduced success rates in:
- Individuals who have undergone radiotherapy
- Turner syndrome (likely due to uterine or endometrial effects)
- Better outcomes in: premature ovarian failure with anatomically normal uterus
Egg-Sharing Programmes
- Women share proportion of retrieved oocytes in return for subsidised fertility treatment
- All gamete donors and recipients undergo counselling prior to treatment
UK Regulations
- Donors cannot be financially remunerated beyond reasonable expenses
- Legal parents decide whether to inform offspring about donor conception
(Buster et al, 1998; HFEA, 2025; Muñoz et al, 2015; Yeh et al, 2014)
—
Topic 19: Sperm Donation
Use of donor sperm in ART has increased over recent years (both IVF and IUI).
Indications
- Azoospermia / severe male-factor infertility not amenable to treatment
- Prior failed ART attributable to male factor
- Heritable genetic disease where donor sperm avoids transmission
- Couples serodiscordant for sexually transmissible viral infections
- Women without a male partner (single women and same-sex couples)
- Couples incompatible for red cell antigens (e.g., D, Kell) associated with haemolytic disease of the newborn with history of severely affected infant
Statistics
- Children born using donor sperm: tripled between 2006 and 2019
- Mainly driven by increasing use among single women and same-sex female couples
- IVF with donated sperm/eggs: 13% NHS funded (vs ~40% for IVF without donation)
- Over 50% of new sperm donors in UK from imports (2020): 27% USA, 21% Denmark
HFEA Regulation and Legality
Age Restrictions:
- Minimum age: 18 years
- Advised under 46 years
- Older donors only in extraordinary circumstances
Donor Anonymity:
- Abolished in 2005
Donor Payments:
- Commercial compensation prohibited in UK
- Reimbursement for justifiable expenses and inconvenience authorised
Compulsory Donor Evaluation:
- Testing for: HIV, Hepatitis B, Hepatitis C, CMV
- Genetic history and risk factors assessed
- Pre-donation counselling and identity verification
Information Access for Donor-Conceived Individuals:
- Prior to age 16: non-identifying information
- At age 16: information regarding donor siblings
- At age 18: identifiable donor information from HFEA
Family Limit per Donor:
- Maximum 10 families per donor
- No cap on number of children per family
Legal Parentage:
- Donors have NO legal rights or obligations for children created through licensed clinics
- Recipients are acknowledged as legal parents
Success Rates (2013 data)
- Total cycles of DI: 4,624
- Births: 590
- Live birth rates:
- Stimulated DI: 14.7%
- Unstimulated DI: 11.2%
All couples should be offered independent counselling covering medical, emotional, and legal implications.
—
Topic 20: Embryo Cryopreservation
Embryo cryopreservation is a proven method of fertility preservation. First human pregnancy following transfer of cryopreserved embryos described in 1983.
Purposes
- Storing supernumerary embryos from initial egg collection/fertilisation
- For subsequent attempt if fresh embryo transfer doesn’t result in live birth
- For further children
- Increases potential embryo replacement cycles without additional egg retrievals
- Improves overall pregnancy rate
- Decreases risk of OHSS
Factors Affecting Success
- Embryo quality – most significant impact on post-thaw survival
- Methods of embryo freezing
- Protocols for post-thaw embryo selection
- Culture conditions
- Patient age at oocyte retrieval
- Number of embryos available for cryopreservation
- Whether previous cycles resulted in pregnancy
Vitrification (Rapid Freezing)
- Survival rates for high-quality blastocysts frequently exceed 90%
- Changes in embryo quality after thawing (re-expansion/”upscoring”) – strong predictor of live birth (Bergin et al, 2024)
Success Rates
- Average live birth rate per FET in UK (2023): approximately 33%
Re-cryopreservation
- Freezing again after thawing associated with lower survival and live birth rates
- Cautious approach recommended
Long-Term Storage
- Has NOT been shown to reduce embryo viability
- UK legislation (since 1 July 2022): Storage permitted for up to 55 years maximum
- Renewal of consent required every 10 years
Obstetric Outcomes
Pregnancies from cryopreserved embryos are comparable to fresh embryo transfers regarding:
- Birth weight
- Gestational age at delivery
- Perinatal mortality
- Rates of major congenital malformations
(Roque et al, 2013; El Bahja et al, 2013; HFEA, 2023)
—
Topic 21: Oocyte Cryopreservation
Indications
Medical:
- Prior to gonadotoxic treatment (chemotherapy, pelvic radiotherapy)
- High risk of premature ovarian insufficiency (POI) due to:
- Genetic factors (Turner’s syndrome mosaicism)
- Medical factors (severe autoimmune conditions, endometriosis)
Non-Medical (Elective/”Social Egg Freezing”):
- Delay childbearing while keeping reproductive options open
- Sperm not available on day of egg collection
- To avoid excessive creation of embryos for ethical, legal, or religious reasons
UK Statistics
- Egg-freezing cycles: 2,567 (2019) → 6,932 (2023) (~170% rise)
- Egg freezing constituted ~7% of all fertility treatment and storage cycles (2023)
Social Context
- Significant increase in elective egg freezing for non-medical reasons
- Associated with:
- Delayed childbearing for financial, educational, or career options
- Growing awareness of fertility
- Wider acceptance of fertility preservation as empowering personal choice
- Not funded by NHS – usually self-funded
Evidence
- Return-to-use rates remain low
- For many women, egg freezing represents reassurance and choice rather than guaranteed plan for motherhood
- Freezing eggs is NOT a guarantee of future pregnancy
Counselling
Paramount in discussing:
- Success rates
- Long-term storage
- Psychological and social contexts associated with elective freezing
- Many women may never need or use their frozen eggs
(Cobo et al, 2016)
—
Topic 22: Gamete and Embryo Cryopreservation in Cancer Therapy
Fertility preservation is an integral part of cancer care for both male and female patients. Survival rates are continuing to improve, and cryostorage has become a realistic option regardless of diagnosis and treatment.
NICE Quality Statement (QS73, 2023)
People of reproductive age with cancer whose treatment is likely to impair fertility should be offered cryopreservation of gametes or embryos before cancer treatment starts.
Multidisciplinary Team Approach
Team should include:
- Oncology specialists
- Surgery specialists
- Fertility specialists
- Mental health specialists
To tailor the plan to the patient’s specific diagnosis, treatment schedule, age, and personal preferences.
Informed consent essential – discussing risks, handling of stored biological material, and potential future outcomes.
Options by Gender (ESHRE, 2020)
Male Patients:
| Option | Notes |
|---|---|
| Sperm banking (semen cryopreservation) | Most commonly used; effective and non-invasive for postpubertal males |
| TESE (testicular sperm extraction) | When ejaculation not possible or patient is azoospermic |
| Testicular tissue cryopreservation | For prepubertal males; considered experimental |
Female Patients:
| Option | Notes |
|---|---|
| Embryo cryopreservation | Well-established; with partner or donor sperm; age-related success rates |
| Oocyte cryopreservation (egg freezing) | Increasingly offered; preferred for women without partner or who don’t want to use sperm |
| Ovarian tissue cryopreservation (OTC) | For patients who cannot defer treatment for ~2 weeks ovarian stimulation; for prepubertal girls; tissue surgically removed and may be reimplanted later |
| Ovarian transposition | Surgically relocating ovaries away from pelvic radiation field; for patients having pelvic radiotherapy |
—
Topic 23: Key Points – Cryopreservation Trends
- Almost no increase in the number of fresh IVF cycles, but a substantial and sustained increase in frozen IVF cycles
- Two-thirds of women having treatment were aged 37 years and under
- Continued increase in IVF cycles using donated sperm or donated embryos (sperm and eggs), but NOT donated eggs
- Patients over 45 years of age are using donated eggs more often than their own
Module 7: Risks and Complications
Topic 1: Risks and Complications of ART – Overview
Morbidity and mortality rates directly related to IVF are low.
Complications predominantly due to:
- Hormonal stimulation
- Egg retrieval
Specific complications include:
- Ovarian hyperstimulation syndrome (OHSS)
- Thromboembolism
- Infection
- Abdominal bleeding
- Adnexal torsion
- Allergic reaction
- Anesthetic complications
If IVF is successful, woman is at risk of usual pregnancy-related morbidity/mortality:
- Preeclampsia/eclampsia
- Hemorrhage
- Thromboembolism
- Sepsis
- Amniotic fluid embolism
Categories of Risk
1. Maternal Risk:
- Psychological effects
- Operative complications
- Ovarian hyperstimulation syndrome (OHSS)
- Risk of cancer
2. Pregnancy-Related Risks:
- Antenatal and delivery complications
- Multiple pregnancy
- Problems of multiple pregnancy and birth
3. Risks to the Offspring:
- Low birth weight (LBW)
- Cardiovascular changes in offspring
- Metabolic outcomes in offspring
- Epigenetic modifications
—
Topic 2: Psychological Effects
Psychological and emotional effects are present before, during and after treatment and can adversely affect the couple’s relationship.
Concerns Include:
- Short-term concerns on outcome of treatment
- Effects on the baby
- Multiple pregnancy
- Long-term emotional consequences of treatment failure
- Risk of cancer
Key Points:
- Association of stress and outcome is more pronounced in the woman
- Critical to find ways of addressing these issues
- Not all can be dealt with via counselling
- Requirement: All patients undergoing assisted reproduction must have access to a counsellor
—
Topic 3: Ovarian Hyperstimulation Syndrome (OHSS)
The most serious complication associated with ART.
Incidence:
- Mild OHSS: 33% of ART cycles
- Severe OHSS: 3.1–8.0% of cycles
Pathophysiology
- Systemic disease resulting from vasoactive products released by hyperstimulated ovaries
- Characterised by increased capillary permeability
- Leakage of fluid from vascular compartment
- Third-space fluid accumulation
- Intravascular dehydration
Symptoms
- Abdominal bloating
- Abdominal pain
- Extreme thirst
- Nausea and vomiting
- Anuria/oliguria
- Shortness of breath
Reference: RCOG Green-top Guideline: Management of Ovarian Hyperstimulation Syndrome
Risk Factors for OHSS
- Young age
- Polycystic ovarian disease
- Diabetes
- Previous OHSS
- High follicular phase LH
- High-dose gonadotrophin stimulation regimens
- Use of GnRH analogues (as opposed to GnRH antagonists)
- Multiple follicular response with stimulation
- High serum estradiol levels during treatment (>20,000 pmol/l)
- Exposure to hCG (as trigger or luteal support)
- Conception (increased in multiple pregnancy)
Prevention Strategies
- Using low-dose stimulation protocols, or natural-cycle IVF
- Follicular monitoring
- Utilising GnRH antagonist cycles rather than GnRH analogues
- Utilising progesterone instead of hCG for luteal support
- Abandoning ART cycles prior to hCG administration and oocyte collection
- Delaying embryo transfer and elective freezing of all embryos
- Coasting: hCG trigger withheld until serum estradiol levels return to acceptable levels
- GnRH agonist triggering (but pregnancy rates are reduced)
Management
- Usually performed on outpatient basis
- Hospital admission for more severe cases (close monitoring of symptoms, biochemistry, fluid balance)
- Occasionally invasive central monitoring in HDU setting required
—
Topic 4: Risk of Cancer
Ovarian Cancer
- Fertility drugs associated with development of ovarian cysts
- Key finding: Infertility itself (by reducing successful pregnancies) is an important risk factor for ovarian cancer
- Fertility treatment does NOT independently increase ovarian cancer risk
- Literature largely reassuring but not definitive
IVF and Ovarian Malignancy
- Whether IVF increases risk (repeated ovarian punctures or other mechanisms) remains controversial
- Overall data are generally reassuring
- No convincing evidence of increased risk of invasive ovarian tumours following fertility drug treatment
- Some studies suggest possible increased risk of borderline ovarian tumours in subfertile women treated with IVF
Breast Cancer
- IVF does NOT appear to increase long-term risk of breast cancer (van den Belt-Dusebout et al, 2016; Reigstad et al, 2015)
Endometrial Cancer
- IVF does NOT appear to increase risk of endometrial cancer (Galbaya, 2010)
(Galbaya, 2010; Rizzuto et al, 2013)
Module 8: Pregnancy-related Complications
Topic 1: Complications in Early Pregnancy
Conception by IVF is associated with an increased incidence of several obstetrical and perinatal complications. Most of these are related to the high incidence of multiple gestations. Singleton pregnancies after IVF are also associated with an increased incidence of complications.
Potential Factors for Increased Adverse Outcomes
The precise reasons for this increase in adverse outcomes are not clear, but potential factors include:
- Maternal and paternal characteristics
- Underlying medical conditions associated with subfertility and infertility
- Sperm factors
- The use of fertility medications
- Laboratory conditions during embryo culture
- Culture medium
- Cryopreservation and thawing
- Prenatal genetic diagnosis (if performed)
- Differences in obstetrical management
- Increased proportion of multiple gestations and vanishing twins
- A combination of these factors
The effects of advanced maternal age also need to be considered since many women who undergo IVF are older and these women are more likely to have pregnancy complications (Kurinczuk & Hockley, 2010).
Early Loss
Early spontaneous pregnancy loss is common in both naturally conceived pregnancies and those conceived using assisted reproductive technologies (ART).
Key Statistics:
- In studies using serial ultrasound examinations of very early IVF pregnancies:
- Spontaneous loss of at least one gestation occurred in approximately 25% of singleton pregnancies
- 35% of twin pregnancies
- 55% of triplet pregnancies
The rate of second-trimester pregnancy loss does not appear to be significantly affected by ART (Kurinczuk & Hockley, 2010).
Ectopic Pregnancy
- Approximately 0.7% of ART cycles result in an ectopic pregnancy
- Higher risk observed in individuals with tubal factor infertility
- Standard IVF with transcervical embryo transfer was associated with an ectopic pregnancy rate of 2.2%
- Embryo transfer to a gestational surrogate was associated with a lower risk of 0.9%, comparable to rates seen in spontaneous conceptions
Heterotopic Pregnancy
Heterotopic pregnancy is considerably more common following ART than in spontaneous conceptions:
- 1 in 100 pregnancies following ART
- Compared with 1 in 30,000 in spontaneous conceptions
This reflects the increased likelihood of multiple gestations following the transfer of more than one embryo (Clayton et al, 2006).
—
Topic 2: Antenatal and Delivery
Several studies have shown increased obstetric morbidity and prenatal morbidity and mortality.
Meta-Analysis Findings
A meta-analysis of 15 singleton pregnancy studies encompassing 1.9 million spontaneous and over 12,000 IVF pregnancies showed significantly increased rates of:
- Perinatal mortality
- Preterm delivery
- Low birth weight
- Neonatal intensive care unit admissions
- Placenta praevia
- Gestational diabetes
- Pre-eclampsia
…for IVF pregnancies.
Multiple Pregnancy Impact
High multiple pregnancy rate following IVF is a significant contributor to these adverse obstetric and perinatal outcomes; hence, the HFEA guidance on reducing the number of embryos transferred to two only. The policy change to that of two-embryo transfer has led to a nine-fold reduction in the use of neonatal intensive care unit services.
Cryopreservation Effects
Available data on the effects of cryopreservation of embryos did not indicate any apparent negative impact on perinatal outcome.
Retrospective Study Findings:
- Compared babies born from cryopreserved embryos (n = 283) with babies born after conventional IVF (n = 961)
- No difference in the incidence of:
- Twins
- Triplets
- Mean gestational age
- Birth weight
- Perinatal mortality rates
Intrapartum complications affect singletons as well as twins, especially those born by caesarean section (Jackson et al, 2004).
—
Topic 3: Multiple Pregnancy
Multiple births are the single biggest risk to the health and welfare of mothers and children born after IVF.
Incidence of Multiple Births After IVF/ICSI
Multifetal pregnancy rates following ART range from 5% to 40%. Dichorionic twin pregnancies are the most common form of multiple gestation following ART.
Monozygotic and Monochorionic Pregnancy Rates:
- IVF population: estimated at 0.9-2%
- Spontaneous conceptions: 0.4%
Higher Order Pregnancies:
- Triplet or more pregnancies in approximately two thirds of cases are the result of ovulation induction without the use of IVF or any similar procedure
Blastocyst Transfers
Blastocyst transfers contribute to the increase of multiple gestations, as embryo splitting at this stage can occur in 6% of cases.
Multiple Gestation Risk by Transfer Type:
| Transfer Type | Multiple Gestation Risk |
|---|---|
| Single blastocyst stage embryo transfer | <2% |
| Double blastocyst embryo transfer | ~39% |
| Double cleavage stage embryo transfer | ~27% |
—
Topic 4: Problems of Multiple Pregnancy and Birth
Twin pregnancies carry much higher obstetric risks for women.
Maternal Complications
The following are all much more common in women carrying twins:
- Miscarriages
- Pre-eclampsia
- Gestational diabetes
- Haemorrhage
- Instrumental deliveries
Fetal/Neonatal Risks
The biggest risk factor for twins is prematurity and low birthweight, which often necessitates hospitalisation, and is linked to:
- Significant risk of neonatal death of one or both twins
- Longer term health and cognitive effects
Twins are at least six times more likely than singletons to suffer from cerebral palsy.
Family Impact
There are also well documented problems for families of twins that range from:
- Financial hardship
- Higher incidence of maternal depression
- Marital problems
—
Topic 5: Elective Single-Embryo Transfer for Good-Prognosis IVF
The only way to reduce the multiple birth rate after IVF is to transfer only one embryo to those women at most risk of having twins.
Key Findings
International datasets show that single-embryo transfer policies can be introduced without significantly reducing pregnancy rates by:
- Targeting good prognosis patients (e.g., relatively young women and women who have not had a number of previous failed IVF attempts)
- Ensuring effective embryo freezing programmes
(Kosmas et al, 2008; Cutting et al, 2008)
Expert Group Recommendations
The decision as to how many patients should receive one embryo is a balance between twin rate and success in achieving a pregnancy.
Based on large national data sets, the expert group on multiple births after IVF concluded that:
- Offering elective single-embryo transfer (eSET) to around 50% of IVF patients will lead to a twin rate of less than 10%
- This is an acceptable balance between reducing the number of twins born after IVF and maintaining IVF patients’ chances to conceive
(Cutting et al, 2008)
Importance of Cryopreservation
It is important that effective cryopreservation programmes accompany eSET, such that:
- Good quality additional embryos are frozen
- Available for transfer should the eSET cycle be unsuccessful
Where more than one good-quality blastocyst is available for transfer on day 5 or 6 of culture, the case for single blastocyst transfer is overwhelming.
eSET is now widely practiced in the UK especially with blastocyst transfers and this has significantly contributed to reduce the multiple pregnancy rates (Khalaf et al., 2008; Cutting et al., 2008).
Policy Options
An expert group has identified two main policy options, both based on the assumption that eSET cycles would be the usual practice for IVF:
- Set clinics an overall maximum proportion of twin births that should not be exceeded (e.g., 5–10%)
- This can be achieved stepwise over time by setting criteria for the group of patients (those with the best prognosis and, thus, the highest risk of multiple pregnancy) who should be offered eSET cycles in the first instance
(Kosmas et al, 2008; Cutting et al, 2008)
Module 9: Perinatal and Paediatric Outcome
Topic 1: Perinatal and Paediatric Outcome Overview
Preterm Birth, Low Birth Weight (LBW) and Small for Gestational Age (SGA)
Singleton IVF pregnancies, with or without ICSI, are at higher risk of preterm birth and low birth weight (LBW) (≤2500 g) compared to spontaneously conceived pregnancies.
Meta-analyses Findings:
- Comprising several thousand IVF cycles and approximately two million spontaneously conceived singleton births
- Matched for maternal age and parity
- Found IVF pregnancies were at significantly higher risk of preterm and small for gestational age (SGA) infants
Frozen Embryo Transfers (FETs):
- From many studies appears that FETs are associated with a decrease in SGA and LBW births, as well as with preterm births
- This suggests that the more natural endometrial preparation prior to FET plays a role in these parameters
- Possibly by allowing for more natural placentation than that which occurs in stimulated cycles
(Jackson et al, 2004; Helmerhorst et al, 2004)
Neurodevelopmental Issues
Neurodevelopmental deficit outcomes include:
- Psychomotor development
- Cognitive development
- Behavioral development
- Socio-emotional development
- Mental disorders (e.g., mental retardation, autism, attention deficit/hyperactivity disorder)
Key Finding: The evidence suggests that the neurodevelopmental outcomes of singleton children conceived after medically-assisted reproduction are similar to those of children conceived naturally (HFEA, 2009).
Pubertal Development
There has been a single report of seven children (six girls and one boy) age 5 to 21 months referred to a pediatric endocrine department because of premature thelarche and pubarche. Sex steroid levels were in the normal prepubertal range and ultrasound examinations of the gonads and adrenals were normal. All of the children were products of ART; however, there was no analysis as to whether the association with ART was higher than expected. The abnormality has not been previously reported. Further study is required before pseudoprecocious puberty can be considered a possible outcome of ART.
ICSI-Conceived Teenagers Study:
A longitudinal study of a cohort of ICSI-conceived teenagers compared pubertal development at age 14 years with a spontaneously conceived control group:
- Menarche: comparable in both groups
- Genital development in boys: comparable in both groups
- Pubic hair development in boys and girls: comparable in both groups
- Breast development: less advanced in ICSI girls compared with spontaneously conceived peers, even after adjustment for known potential confounders
(HFEA, 2009)
—
Topic 2: Congenital Anomalies
IVF/ICSI and Birth Defects
In a systematic review of 25 studies, seven of which were included in a meta-analysis, comparing IVF/ICSI and spontaneous pregnancies:
Key Finding: There was a statistically significant increased risk (OR: 2.02; 95% CI: 1.49–2.69) of birth defect by 30–40% following ART.
Other findings:
- Increased risk for IVF/ICSI
- The risk is greater for ICSI than for IVF, especially for urogenital problems
(Wen et al, 2012; Heisey et al, 2015; Hansen et al, 2005)
Cryopreservation Effects
Available data did not indicate any apparent negative effects of cryopreservation of embryos on early infant development or congenital malformation rate.
Retrospective Study:
- Compared babies from cryopreserved embryos with babies after conventional IVF
- Incidence of major congenital malformations was significantly lower in the cryopreserved group (1%) than in the IVF group (3%)
Matched Study:
One study matched:
- 255 children from cryopreserved embryos
- 255 children born after standard IVF with fresh embryos
- 252 children from spontaneous pregnancies
Matched for: maternal age, parity, single or twin pregnancy and date of delivery
Results at 18 months:
- Growth: similar in all three groups
- Incidence of major malformations: similar in all three groups
- Prevalence of chronic diseases: similar in all three groups
(Bonduelle et al, 2005)
—
Topic 3: Risk of Cancer and Other Outcomes in Offspring
Cancer in Offspring
A 2013 systematic review and meta-analysis including 25 case-control and cohort studies reported an association between fertility treatment and cancer in the offspring. Fertility treatment included any medically assisted reproduction procedure that involved ovulation induction (e.g., controlled ovarian stimulation, ovulation triggering, intrauterine insemination, assisted reproductive technology (IVF and ICSI)) (Hargreave et al, 2013).
Key Uncertainty: It remains unclear whether the risks observed are related to one or more aspects of fertility treatment itself or to personal factors associated with or causing subfertility. Only two small studies in the meta-analysis used an untreated sub-fertile group of women as a control group and neither reported a statistically significant association between fertility treatment and cancer in their offspring; however, the findings are inconclusive given the small number of cases in these two studies (Raimondi et al, 2005).
Obstetrical Complications
Even among singleton gestations, IVF has been associated with an increased risk of pregnancy complications such as:
- Placenta previa and abruption
- Gestational diabetes
- Preeclampsia
- Cesarean delivery
However, the absolute increase in risk has generally been small and most such pregnancies have normal outcomes.
Frozen Embryo Transfers (FETs):
- There is a suggestion that FETs are associated with decreased risk of placenta previa and abruptio placentae
- This suggests that the endometrial environment at the time of implantation plays a role in the pathogenesis of these complications
Preeclampsia Study:
A cohort study of over 500,000 women attempted to determine the risk of preeclampsia in women undergoing ART, including IVF, compared with women who had a natural conception. After adjusting for maternal age, interbirth interval, and presence of new partner, women undergoing ART had a small increase in odds of preeclampsia in their second and third pregnancies compared with women who did not require ART.
Perinatal Mortality
Stillbirth and perinatal mortality rates appear to be increased as much as four-fold due, at least in part, to:
- Obstetrical complications
- Low birth weight (LBW)
- Multiple gestation
Other Long-term Issues
- Hospitalisations appear to be more common for ART children when compared with those not conceived by ART
- However, the overall incidence of chronic illness is not increased even when outcomes among ART offspring up to 28 years of age have been reported
- Such data are generally reassuring for the parents
—
Topic 4: Key Points
Patient Counselling:
- Patients should be informed that pregnancies conceived by ART are associated with increased risks of:
- Multiple gestations
- Congenital anomalies
- Preterm delivery
- Low birth weight (LBW)
- The complications associated with these outcomes
- Even for singleton ART pregnancies, the relative risk of common pregnancy complications such as:
- Fetal growth restriction
- Preeclampsia
- Prematurity
- Perinatal mortality
…are increased, although not clearly independent of infertility itself.
RCOG Assisted Reproduction Technology (ART) – Complete Assessment Guide
Course Overview
- Course: Assisted Reproduction
- Provider: Royal College of Obstetricians and Gynaecologists (RCOG)
- Final Score: 100% on all assessments
—
Assessment 1 – Assisted Reproduction (5/5 – 100%)
Question 1
Scenario: A couple where the male partner is healthy and the female partner suffers from Turner’s syndrome (XO)
Correct Answer: In vitro fertilisation using donor oocytes
Feedback: The answer is in vitro fertilisation using donor oocytes.
—
Question 2
Scenario: A couple with no male factor problem but the female partner has polycystic ovarian syndrome not responding to clomifene and metformin therapy
Correct Answer: In vitro fertilisation using own gametes
Feedback: The answer is in vitro fertilisation using own gametes.
—
Question 3
Scenario: A couple with unexplained infertility
Correct Answer: In vitro fertilisation using own gametes
Feedback: The answer is in vitro fertilisation using own gametes.
—
Question 4
Scenario: A couple where the female partner has pelvic endometriosis affecting the tubes and the male partner has severe oligo-astheno-spermia and is a carrier of cystic fibrosis gene mutation
Correct Answer: Intracytoplasmic sperm injection (ICSI)
Feedback: The answer is intracytoplasmic sperm injection. This can be done with the woman’s own egg, provided she is not a carrier of CF.
—
Question 5
Scenario: A couple where the female partner has complete Rokitansky syndrome with right renal absence
Correct Answer: Surrogacy
Feedback: The answer is surrogacy. In Rokitansky syndrome, the uterus is missing and not ovaries so patient can use her own eggs.
—
Final Assessment 1 (25/25 – 100%)
Question 1
Question: NICE recommended BMI of the female partner before commencing fertility treatment
Correct Answer: 19-30
—
Question 2
Question: Frozen-thawed embryo replacement increases the overall cumulative pregnancy rate by approximately ____
Correct Answer: 11%
—
Question 3
Question: Oocyte retrieval is carried out _____ hours after the hCG injection
Correct Answer: 34-37
—
Question 4
Question: At the age of 40 years, the woman’s risk of miscarriage per IVF pregnancy
Correct Answer: 23%
—
Question 5
Question: What proportion of pregnancies following transfer of two blastocysts during IVF are twins
Correct Answer: 35%
—
Final Assessment 2 (4/4 – 100%)
Question 1
Question: Inseminated oocytes are checked for signs of fertilisation after approximately ____ hours
Correct Answer: 18
—
Question 2
Question: Optimal endometrial thickness in millimetres for frozen-thawed embryo replacement cycles
Correct Answer: 7-11
—
Question 3
Question: Undertaking eSET in approximately 50% of IVF patients will reduce the multiple twin birth rate to less than ____
Correct Answer: 10%
—
Question 4
Question: Up to ___ of women with moderate/severe OHSS may require admission to hospital
Correct Answer: 1%
—
Final Assessment SBAs (8/8 – 100%)
Question 1
Question: The most important initial investigation for him is to have: (regarding severe oligospermia with sperm concentration <5 x 10⁶/ml)
Correct Answer: A Karyotype
Feedback: A Karyotype should be performed in men with azoospermia and severe oligozoospermia (sperm concentration of <5 x 10⁶/ml). The most common karyotypic abnormality is Klinefelter syndrome; other sex chromosomal aberrations may also be seen. Repeating the semen analysis when he has had two tests is not useful.
—
Question 2
Question: What is the most appropriate management option? (regarding bilateral tubal disease)
Correct Answer: Recommend laparoscopic salpingectomy prior to IVF
Feedback: IVF should be considered as the first-line treatment for moderate-to-severe bilateral tubal disease. A systematic review has concluded that laparoscopic salpingectomy should be considered prior to IVF as this significantly raises live birth rate (72% with two to three cycles of treatment) compared with 24% following tubal surgery.
—
Question 3
Scenario: A couple were investigated for infertility and the man indicated a concentration of 11 million per ml, 6% progressive motility and 1% normal forms. The woman had unilateral tubal blockage at diagnostic laparoscopy and dye test.
Question: What is the most appropriate management option?
Correct Answer: Intracytoplasmic sperm injection (ICSI)
Feedback: Intracytoplasmic sperm injection should be considered as first line of treatment with severe sperm abnormalities as per NICE 2013 recommendations.
—
Question 4
Scenario: A couple attended fertility unit for investigations for primary sub-fertility. A diagnostic laparoscopy and dye test on the woman showed moderate endometriosis and forced filling and spillage of the dye in both tubes. The sperm count was found to be 1 million/ml.
Question: What is the most appropriate management option?
Correct Answer: Intracytoplasmic sperm injection (ICSI)
Feedback: ICSI is recommended by NICE 2013 as the first line of treatment, due to severe oligospermia whether obstructive or non-obstructive causes.
—
Question 5
Scenario: A 40-year-old woman presents with primary subfertility for 3 years. On examination, her pelvis was normal on laparoscopy and dye test, and her day 21 serum progesterone is 40 pmol/l. Her partner’s semen analysis is volume of 3ml; sperm count of 40 million/ml, white cell count 0.5 million/ml; 80% motile sperm. She enquires about her chances of successful pregnancy using IVF.
Question: What is the most appropriate answer?
Correct Answer: 20%
Feedback: In 2014, a trial that randomized 154 couples with ≥6 months of unexplained infertility and female partner aged 38 to 42 years of age to two cycles of clomiphene/IUI, two cycles of gonadotropin injections/IUI, or immediate IVF, live birth rates were: 8, 7, and 16 percent, respectively.
Reference: Goldman MB, Thornton KL, Ryley D, et al. A randomized clinical trial to determine optimal infertility treatment in older couples: the Forty and Over Treatment Trial (FORT-T). Fertil Steril 2014; 101:1574.
—
Question 6
Scenario: A 32-year-old woman is requesting reversal of sterilisation. The sterilisation was performed three years ago using Filshie clips. She has two children, but is now in a new relationship. Her partner’s semen analysis is normal.
Question: What are her chances of pregnancy after reversal of sterilisation?
Correct Answer: 55-85%
Feedback: Most studies showed pregnancy rates after sterilization reversal among women aged 15 to 30 years, 30 to 33 years, and 34 to 49 years were 73, 64, and 46 percent, respectively. Most pregnancies occurred within two years after reversal. Also, tubal anastomosis resulted in live births in 41 percent of women with a previous electrocautery procedure, 50 percent of those who had a Pomeroy tubal ligation, 75 percent of women with rings, and 84 percent of those with clips.
—
Question 7
Scenario: A 30-year-old couple presents with primary sub-fertility for 3 years due to unexplained infertility.
Question: What is the most appropriate management?
Correct Answer: IVF
Feedback: IVF treatment is recommended by NICE as first line of treatment for unexplained sub-fertility more than 2 years.
—
Question 8
Scenario: A 37-year-old woman has Turner syndrome presented with primary subfertility. She has cyclical bleeding with HRT. Semen analysis of her partner was normal.
Question: What is the most appropriate management?
Correct Answer: IVF with donor oocytes
Feedback: Women with Turner syndrome can carry a pregnancy after in vitro fertilization (IVF) using donor oocytes. The endometrial response of these patients to adequate estrogen and progestin treatment appeared to be normal. The rate of successful pregnancy with this technology is similar to that for women without Turner syndrome.
—
Assessment: Part 3 – IUI Suitability (1/1 – 100%)
Question 1
Scenario: A test wash was performed to discover the suitability of the sperm sample for intrauterine insemination (IUI). The sample had 8 million motile sperm per ml. The hysterosalpingogram confirmed bilateral patent tubes.
Statement: This couple is suitable for IUI
Correct Answer: True
Feedback: The answer is true. Generally, there is a consensus that at least 5 million motile sperm per ml on a prepared sample is suitable for IUI.
—
Assessment: Part 4 – Prognostic Factors (2/2 – 100%)
Question 1
Question: Regarding prognostic factors for assisted reproduction techniques, which one of the following statements is incorrect?
Options:
- A maternal BMI of 19-29 is associated with a higher chance of success
- A previous successful pregnancy (spontaneous or assisted) improves the chance of success
- Duration of infertility has no effect on success rates ← CORRECT (This is the INCORRECT statement)
- The chance of a successful pregnancy is reduced in women >35 years of age
- The highest success rates are seen in the first three cycles of treatment
Feedback: The answer is duration of infertility has no effect on success rates. The longer the duration of infertility, the lower the chances of success with assisted reproduction techniques.
—
Question 2
Question: Which of the following treatment options would normally now be recommended to this couple?
Correct Answer: In vitro fertilization
Feedback: The answer is in vitro fertilization.
—
Assessment: Part 5 – OHSS (2/2 – 100%)
Question 1
Question: Which one of the following is NOT a risk factor for ovarian hyperstimulation syndrome?
Correct Answer: BMI >30
Feedback: The answer is a BMI >30. In fact, young slim women are at higher risk of developing ovarian hyperstimulation syndrome.
Risk Factors for OHSS include:
- hCG administration for luteal support
- High serum oestradiol on the day of hCG trigger injection
- Multiple pregnancy
- Polycystic ovarian syndrome
- Young age
—
Question 2
Question: Regarding ovarian hyperstimulation syndrome, which one of the following statements is incorrect?
Correct Answer: Admission to hospital is always indicated
Feedback: The answer is admission to hospital is always indicated. (This is incorrect – not all OHSS cases require hospitalization)
Correct statements about OHSS:
- OHSS occurs in 0.6-10% of IVF cycles
- Thromboprophylaxis is imperative in women with moderate-to-severe OHSS
- Treatment is mainly conservative/supportive
—
Assessment: Part 3 – Tubal Patency (2/2 – 100%)
Question 1
Question: Which of the following would be the preferred option to confirm tubal patency? (Patient with history of chlamydia)
Correct Answer: Laparoscopy and dye test
Feedback: The correct answer is laparoscopy and dye test. Ms MS is at an increased risk of adhesions and tubal pathologies because of the previous history of chlamydia. Therefore, laparoscopy is preferred over hysterosalpingogram as further treatment, such as adhesiolysis, can be performed at the same time.
In this case, laparoscopy and dye test were normal.
Conclusion: A successful pregnancy was achieved after three cycles of treatment with clomiphene citrate.
—
Question 2
Question: Which of the following treatment options would now normally be recommended? (For anovulation with normal tubes)
Correct Answer: Clomiphene citrate
Feedback: Since anovulation is the problem, ovulation induction with clomiphene citrate is appropriate. Clomiphene should be administered for 5 days from day 2-6 of the cycle. The first cycle is monitored with ultrasound to confirm ovulation and to monitor follicle development and reduce the risk of multiple pregnancy. Subsequent cycles can be monitored with midluteal (day 21) progesterone levels.
—
Assessment: Part 4 – Clomiphene Citrate (2/2 – 100%)
Question 1
Question: Which of the following statements regarding treatment with clomiphene citrate is incorrect?
Correct Answer: Approximately 60-70% of women will ovulate
Feedback: The answer is approximately 60-70% of women will ovulate. Actually, approximately 80% of women will ovulate with clomiphene treatment.
Correct statements about Clomiphene:
- Side effects include visual disturbances and hot flushes
- The pregnancy rate after six ovulatory cycles is 50%
- The risk of multiple pregnancy is increased up to eight-fold compared with spontaneous conceptions
—
Question 2
Question: Which one of the following statements regarding clomiphene is incorrect?
Correct Answer: The half-life of the drug is 2 days
Feedback: The answer is the half-life of the drug is 2 days. The half-life is actually 5-7 days.
Correct statements about Clomiphene:
- It increases the production of gonadotrophins (FSH) by inhibiting feedback on the hypothalamus
- It is a selective oestrogen receptor modulator
- It is an antiestrogenic agent
—
Key Learning Points Summary
IVF Indications
- Turner’s syndrome → IVF with donor oocytes
- PCOS not responding to clomifene/metformin → IVF with own gametes
- Unexplained infertility >2 years → IVF (NICE recommendation)
- Rokitansky syndrome → Surrogacy (uterus absent, ovaries present)
ICSI Indications
- Severe oligospermia (<5 million/ml)
- Severe oligo-astheno-spermia
- Male factor with CF carrier status
Important Numbers
| Parameter | Value |
|---|---|
| NICE recommended BMI | 19-30 |
| FET pregnancy rate increase | 11% |
| Oocyte retrieval after hCG | 34-37 hours |
| Fertilisation check | 18 hours |
| Optimal endometrial thickness | 7-11 mm |
| Miscarriage risk at age 40 | 23% |
| Twin rate with 2 blastocysts | 35% |
| eSET reduces twins to | <10% |
| OHSS requiring admission | Up to 1% |
| IUI minimum motile sperm | 5 million/ml |
| Clomiphene ovulation rate | 80% |
| Clomiphene half-life | 5-7 days |
| Sterilisation reversal (clips) | 55-85% pregnancy rate |
Karyotyping
- Required in men with azoospermia or severe oligospermia (<5 x 10⁶/ml)
- Most common finding: Klinefelter syndrome
OHSS Risk Factors
- Young age
- Low BMI (NOT high BMI)
- PCOS
- High oestradiol on trigger day
- hCG for luteal support
- Multiple pregnancy
Tubal Assessment
- History of chlamydia → Laparoscopy and dye test preferred (allows treatment of adhesions)
- Bilateral tubal disease → Consider salpingectomy before IVF (improves live birth rate)
—
Reflective Tasks
Reflective Task 1 – Case Study: Mrs SL & Mr AL
Patient Details:
- Mrs SL (36 years old) and Mr AL (38 years old)
- Trying for baby for just over 2 years
- Neither have had a baby before (primary subfertility)
Mrs SL – History:
- First period at 14 years of age
- Regular cycles (28-31 days)
- Up-to-date cervical smears
- Immune to rubella
- Negative chlamydia swab
- No coital problems
- Asthmatic (used inhaler)
- Non-smoker
- Social alcohol use only
- No known allergies
- Takes folic acid regularly
- BMI: 25
Investigation Results (Mrs SL):
- Day 3 FSH: 6.7 IU/l
- LH: 7.2 IU/l
- Day-21 progesterone: 41 nmol/l
Mr AL – History:
- Fit and healthy
- No significant past medical history
- Non-smoker
- 5-6 units alcohol/week
Semen Analysis Results (Mr AL):
- Volume: 3.0 ml
- Count: 8 million/ml
- Progressive motility: 15%
- Normal forms: 7%
Questions to Reflect On:
- Is the semen analysis normal?
- Based on the patients’ details, which investigations should be conducted?
- Is a repeat semen analysis necessary?
Part 2 – Follow-up:
A repeat semen analysis confirmed oligoasthenozoospermia:
- Concentration: 12 million/ml
- Motility: 50%
Question: Based on the test results, which further investigations should the consultant conduct?
—
MODEL ANSWERS – Reflective Task 1
Q1: Is the semen analysis normal?
- Answer: No. The initial semen analysis shows:
- Count: 8 million/ml (abnormal – WHO reference: ≥15 million/ml)
- Progressive motility: 15% (abnormal – WHO reference: ≥32%)
- Normal forms: 7% (borderline – WHO reference: ≥4%)
- This indicates oligoasthenozoospermia (low count + poor motility)
Q2: Is this couple suitable for IUI?
- Answer: True – After test wash showing 8 million motile sperm per ml with bilateral patent tubes confirmed on HSG
- Model Answer: Generally, there is a consensus that at least 5 million motile sperm per ml on a prepared sample is suitable for IUI
Q3: Regarding prognostic factors for ART, which statement is INCORRECT?
- Answer: “Duration of infertility has no effect on success rates” is INCORRECT
- Model Answer: The longer the duration of infertility, the lower the chances of success with assisted reproduction techniques
Q4: Which treatment would normally now be recommended?
- Answer: In vitro fertilization (IVF)
- Model Answer: Given the male factor (oligoasthenozoospermia) and duration of subfertility (>2 years), IVF is the appropriate next step
Q5: Which is NOT a risk factor for OHSS?
- Answer: BMI >30 is NOT a risk factor
- Model Answer: Young slim women are at higher risk of developing OHSS. Risk factors include: young age, low BMI, PCOS, high oestradiol on trigger day, hCG for luteal support, multiple pregnancy
Q6: Which statement about OHSS is INCORRECT?
- Answer: “Admission to hospital is always indicated” is INCORRECT
- Model Answer: Not all OHSS cases require hospitalization. OHSS occurs in 0.6-10% of IVF cycles, treatment is mainly conservative/supportive, and thromboprophylaxis is imperative in moderate-to-severe cases
—
Reflective Task 2 – Case Study: Ms JM & Mr MS
Patient Details:
- Ms JM (32 years old, Italian dressmaker) and Mr MS (35 years old)
- 1-year history of primary subfertility
Ms JM – History:
- First period at 12 years of age
- Irregular cycles (30-90 days) – suggesting anovulation
- Up-to-date cervical smears
- Immune to rubella
- Negative chlamydia swab (but history of chlamydia treated with antibiotics)
- Regular intercourse 3-4 times/week
- Previous surgery: breast augmentation only
- Smokes 10 cigarettes/day (advise smoking cessation)
- 8-10 units alcohol/week
- No known allergies
- Takes folic acid regularly
- BMI: 22
Investigation Results (Ms JM):
- Day 3 FSH: 8.1 IU/l
- LH: 17.2 IU/l (elevated LH:FSH ratio suggests PCOS)
- Serial progesterone levels (all low – confirming anovulation):
- Day 21: 5 nmol/l
- Day 28: 8 nmol/l
- Day 35: 6 nmol/l
- Day 42: 10 nmol/l
Mr MS – History:
- Fit and healthy
- No significant past medical history
- Non-smoker
- 20-25 units alcohol/week (advise reduction)
Semen Analysis Results (Mr MS):
- Volume: 3.0 ml
- Count: 108 million/ml ✓
- Progressive motility: 56% ✓
- Normal forms: 32% ✓
- Normal semen analysis
Questions to Reflect On:
- Is the semen analysis normal?
- Based on the patients’ details, which further investigations should the consultant conduct?
—
MODEL ANSWERS – Reflective Task 2
Q1: Is the semen analysis normal?
- Answer: Yes. Mr MS has normal semen parameters:
- Count: 108 million/ml (normal – WHO reference: ≥15 million/ml)
- Progressive motility: 56% (normal – WHO reference: ≥32%)
- Normal forms: 32% (normal – WHO reference: ≥4%)
Q2: Is there sufficient evidence to make a diagnosis of PCOS?
- Answer: The clinical picture is highly suggestive of PCOS:
- Irregular cycles (30-90 days) indicating anovulation
- Elevated LH:FSH ratio (17.2:8.1 = 2.1:1)
- Serial low progesterone levels confirming anovulation
Q3: Which investigation is preferred to confirm tubal patency?
- Answer: Laparoscopy and dye test
- Model Answer: Ms JM is at an increased risk of adhesions and tubal pathologies because of the previous history of chlamydia. Therefore, laparoscopy is preferred over hysterosalpingogram as further treatment, such as adhesiolysis, can be performed at the same time
Q4: What treatment would normally be recommended?
- Answer: Clomiphene citrate
- Model Answer: Since anovulation is the problem, ovulation induction with clomiphene citrate is appropriate. Clomiphene should be administered for 5 days from day 2-6 of the cycle. The first cycle is monitored with ultrasound to confirm ovulation and to monitor follicle development and reduce the risk of multiple pregnancy. Subsequent cycles can be monitored with midluteal (day 21) progesterone levels
Q5: Which statement about clomiphene treatment is INCORRECT?
- Answer: “Approximately 60-70% of women will ovulate” is INCORRECT
- Model Answer: Actually, approximately 80% of women will ovulate with clomiphene treatment. Side effects include visual disturbances and hot flushes. The pregnancy rate after six ovulatory cycles is 50%. The risk of multiple pregnancy is increased up to eight-fold
Q6: Which statement about clomiphene is INCORRECT?
- Answer: “The half-life of the drug is 2 days” is INCORRECT
- Model Answer: The half-life of clomiphene is actually 5-7 days. Clomiphene is a selective oestrogen receptor modulator and antiestrogenic agent that increases FSH production by inhibiting feedback on the hypothalamus
CASE CONCLUSION:
- Laparoscopy and dye test were normal in Ms JM’s case
- A successful pregnancy was achieved after three cycles of treatment with clomiphene citrate
—
Key Learning Points from Reflective Tasks:
| Finding | Interpretation |
|---|---|
| Irregular cycles + low progesterone | Anovulation |
| LH:FSH ratio >2 | Suggests PCOS |
| History of chlamydia | Risk of tubal damage – needs laparoscopy |
| Sperm count <15 million/ml | Oligospermia |
| Motility <32% | Asthenozoospermia |
| Smoking | Advise cessation – affects fertility |
| High alcohol intake | Advise reduction |
| Anovulation + normal tubes + normal sperm | First-line: Clomiphene citrate |
| Male factor + female factor | Consider IVF or ICSI |
—
Assessment completed with 100% on all modules

Leave a comment